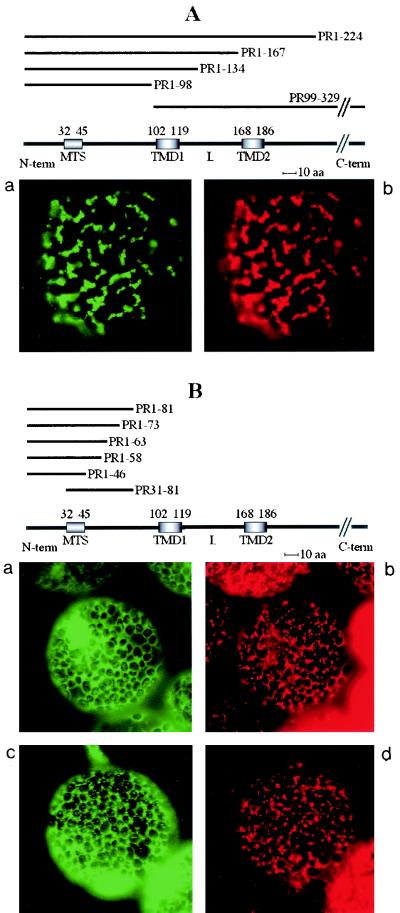
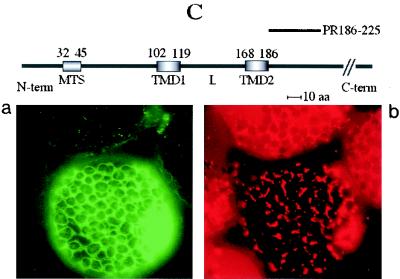
FIG. 6.
Transient expression of GFP fusions in N. benthamiana leaves. Constructs containing the unfused GFP gene, the GFP gene fused to the cDNA encoding the complete 36K or 95K protein, or the GFP gene fused to cDNAs encoding the different deletion variants (PR1-224, PR1-167, PR1-134, PR1-98, PR99-329, PR1-81, PR1-73, PR1-63, PR1-58, PR1-46, PR31-81, and PR186-225) were expressed. Fluorescence was observed at 40× or 100× magnification with a Nikon Eclipse E800 epifluorescence microscope. In each pair of panels, the image on the left shows the GFP fluorescence observed with a GFP band pass filter set (excitation, 460 to 500 nm; band pass emission, 510 to 560 nm), and the right-hand image shows the fluorescence of the mitochondrion-specific dye (MitoTracker) observed for the same cell by using a tetramethylrhodamine isothiocyanate filter set (excitation, 27.5 to 552.5 nm; emission, 577.5 to 632.5 nm). The structures of the deletion mutants yielding the different types of fluorescence pattern are presented above the image sets. The relative positions of the putative MTS (amino acids 32 to 45), the two putative transmembrane domains (TMD1, amino acids 102 to 119, and TMD2, amino acids 168 to 186), and the loop (L) in the ORF1 sequence are indicated. The sequences retained in the different deletion mutants (PRx-y) are indicated by heavy lines. Three different types of subcellular localization of the GFP fusion protein were observed and are illustrated by characteristic pictures. When fused to 95K protein, 36K protein, PR1-224, PR1-167, PR1-134, PR1-98, and PR99-329, the GFP was exclusively localized to mitochondria (A). When fused to PR1-81, PR1-73, PR1-63, PR1-58, PR1-46, and PR31-81, the GFP was diffuse in the cytosol (B, panel a), but additional localization to small structures poorly representative of the mitochondrial staining pattern occurred at a late stage of transient expression (B, panel c). GFP fused to PR186-225 was diffuse in the cytosol, like unfused GFP (C).