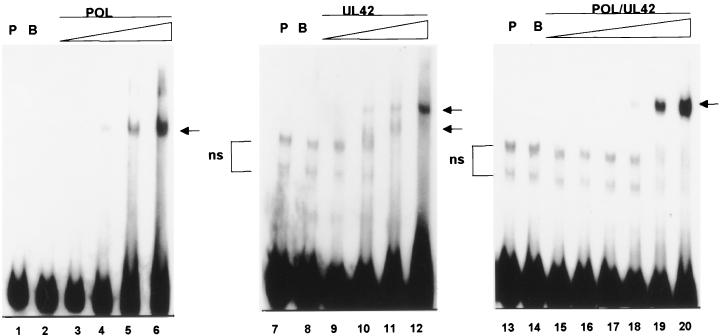
FIG. 2.
Electrophoretic mobility shift analysis of protein binding to model P/T. Purified Pol (lanes 3 to 6), UL42 (lanes 9 to 12), or Pol/UL42 heterodimer (lanes 15 to 20) preparations were incubated with 32P-end-labeled P/T (100 nM), and the formation of complexes was analyzed by electrophoresis through nondenaturing 6% polyacrylamide gels. The final concentrations of Pol and UL42 tested were 100, 200, 300, and 400 nM, while those tested for the Pol/UL42 heterodimer were 25, 50, 100, 200, 300, and 400 nM (concentration shown by the thickness of the triangle over the lanes). The mobility of the P/T probe (P) alone (lanes 1, 7, and 13) or in the presence of 400 nM BSA (B) (lanes 2, 8, and 14) also is shown. In some preparations of probe, nonspecific (ns) higher-mobility bands were noted. Specifically shifted complexes are indicated by arrows.