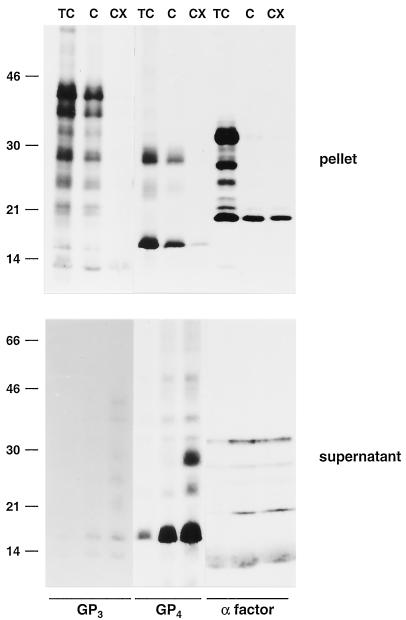
FIG. 3.
Membrane association of the GP4 and GP3 proteins. ORF4 and -3 RNA transcripts were translated in a rabbit reticulocyte lysate in the presence of canine pancreatic microsomes. Equal portions of the in vitro translation mixtures were incubated for 60 to 120 min at 4°C in three different buffers: 50 mM Tris-HCl (pH 7.6)-25 mM CaCl2 (TC), 100 mM sodium carbonate (pH 11.5) (C), and 100 mM sodium carbonate (pH 11.5)-2% TX-100 (CX). After subsequent high-speed centrifugation of the samples, the pellets were dissolved directly in LSB containing 5% β-mercaptoethanol and analyzed in SDS-15% PAA gels while the GP3 and GP4 proteins released into the supernatants were immunoprecipitated with the αGP4E and αGP3 antisera, respectively. The yeast α-factor was used as a soluble-protein control. Since a specific antibody for this protein was not available, the supernatants were not subjected to IP but dissolved directly in LSB containing 5% β-mercaptoethanol. The values on the left are the molecular sizes, in kilodaltons, of marker proteins analyzed in the same gels.