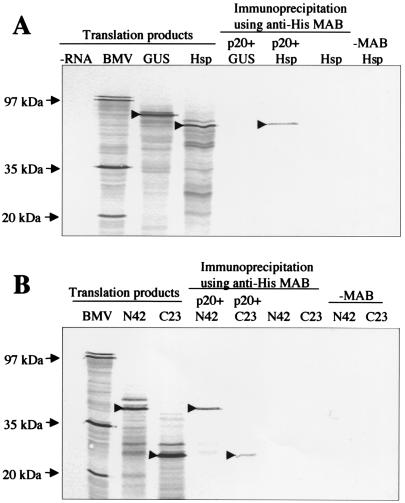
FIG. 3.
Coimmunoprecipitation of the 35S-labeled Hsp70h (A) and N42 and C23 (B) with unlabeled, histidine-tagged p20. For lanes under the heading translation products, the isotope-labeled products of in vitro translation reactions programmed with no RNA (negative control; −RNA lane), Brome mosaic virus RNA (positive control; BMV lane), GUS mRNA (GUS lane), Hsp70h mRNA (Hsp lane), N42 mRNA (N42 lane), or C23 mRNA (C23 lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto a nitrocellulose membrane, and subjected to autoradiography. For lanes under the heading immunoprecipitation using anti-His MAB, the indicated labeled translation products were mixed with histidine-tagged, unlabeled p20 and were immunoprecipitated by using monoclonal, histidine tag-specific antibody and protein A-Sepharose. Negative controls in which p20 or monoclonal antibody were omitted are also shown. The arrows at the left mark the positions of corresponding BMV proteins, whereas the arrowheads within the autoradiograms mark the major translation products of the mRNAs that encode GUS, Hsp70h, N42, and C23 before or after immunoprecipitation.