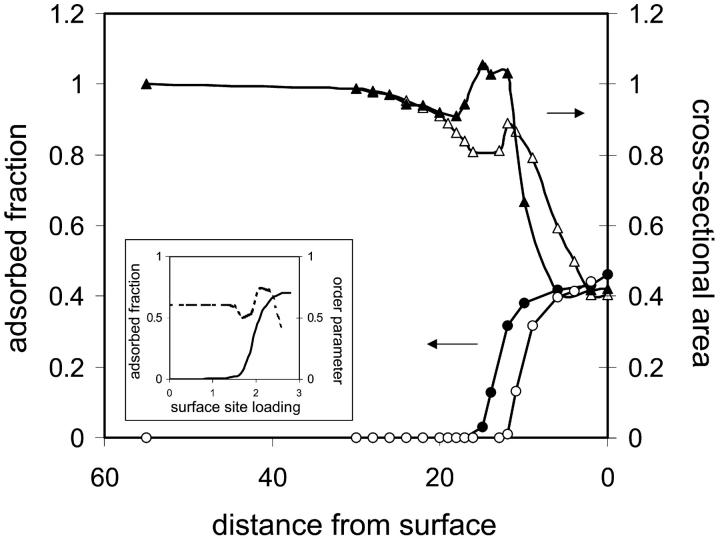
FIGURE 2.
Adsorption isotherms and cross-sectional area changes of the protein as a function of distance from the surface. (Inset) Adsorbed fraction (solid line), and conformation order parameter (dashed line) as a function of surface loading for random heteropolymer (RHP) with strong specific intersegment interactions, reproduced from Srebnik et al. (56). The conformation order parameter accounts for the number of available conformations for the RHP. When it equals unity, the entire conformational space is sampled; when it is less than unity, the RHP are restricted to a small number of energetically favored conformations. For the case of strong specific interactions between the heteropolymer segments and surface, it is seen that a sharp adsorption transition is accompanied first by restricting further the conformational space available for the RHP, followed by unfolding of the RHP and refolding into a surface-matched compact conformation. This situation is akin to adsorption of a protein in its native state, followed by “unraveling” of the folded protein before adsorbing into conformation determined by the interactions with the surface.