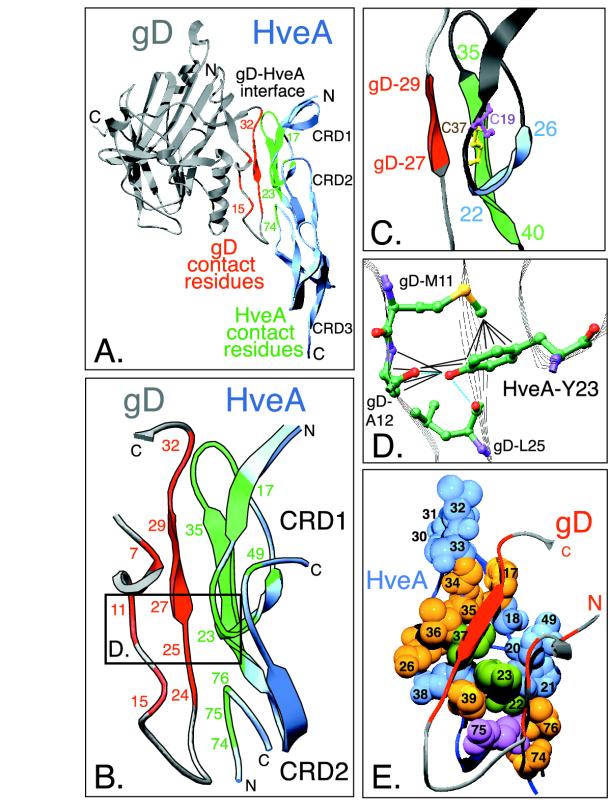
FIG. 2.
Highlighted regions within the gD-HveA crystal structure. (A) Ribbon diagram of the crystal structure of HveA bound to gD. The N- and C-terminal residues observed in the crystal structure and the location of HveA CRDs are indicated. The HveA molecule is shown partially in blue, and the HveA contact residues found within CRD1 and CRD2 are shown in green. The gD molecule is shown in gray, with the contact residues located in the N-terminal loop of gD shown in red. Contact residues were defined as amino acids containing atoms that come within 4 Å of the partner molecule (see Table 1). Some of the contact residues are numbered for reference. (B) An enlarged view of the gD-HveA interface shown in the same orientation as in panel A. HveA contact residues are displayed in green, and gD contact residues are red. The boxed area indicates a region shown at higher magnification in panel D. (C) Magnification of a disulfide bond within HveA CRD1 and three β-strands within the gD-HveA binding site. HveA-C37 (yellow) forms a disulfide bond with HveA-C19 (purple). Residues 35 to 37 within a β-strand of HveA (residues 35 to 40, shown in green) form hydrogen bonds with a short β-strand on gD (gD residues 27 to 29, shown in red) to form an intermolecular, antiparallel β-sheet. This augments a two-stranded β-sheet in HveA CRD1 formed by residues 22 to 26 (blue) and residues 35 to 40 (green). (D) Interactions between HveA-Y23 and gD amino acids. Carbon (green), oxygen (red), nitrogen (purple), and sulfur (yellow) atoms are shown. The blue dotted lines indicate hydrogen bonds between HveA-Y23 and gD. The black lines indicate other interactions, defined as distances of 4 Å or less (see Table 1). The hydroxyl group of HveA-Y23 interacts with gD-A12 and gD-L25. The phenyl ring of HveA-Y23 interacts with gD-M11 and gD-A12. (E) Space-filling model of the gD binding site on the surface of HveA. The gD-HveA interface from panel B is rotated so that the N-terminal loop of gD lies on top of the HveA binding surface. The N-terminal loop of gD is shown as a gray ribbon, with the gD contact residues colored red. The HveA contact residues are numbered. The space-filling spheres represent atoms of HveA contact residues from category 1 (blue), category 2 (purple), category 3 (yellow), and category 4 (green) (see Table 1). HveA-Y23 (green) is located at the center of the interface.