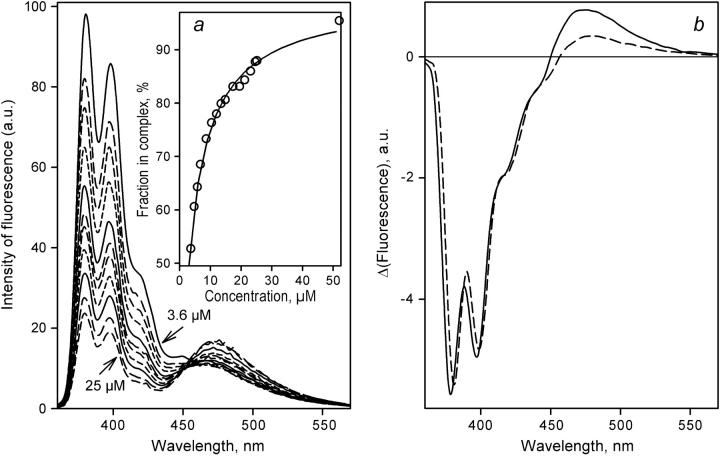
FIGURE 5.
Determination of KD of the complex of labeled P450eryF S93C/C154S with 1-PB bound at the low-affinity binding site by FRET in a dilution setup. (a) Normalized emission spectra of a mixture of P450eryF with 1-PB taken at a 1:3.3 molar ratio at 3.6, 4.7, 5.8, 6.8, 10, 12., 15, 17, 21, 23, and 25 μM P450eryF-MDCC. Intensity of fluorescence is expressed in arbitrary units (a.u.). The inset shows the results of the same experiment as the plot of the amplitude of FRET given by the loading factor of the first principal component versus the concentration of P450eryF-MDCC. The solid line shows the approximation of this data set by the equation for the equilibrium of bimolecular association with KD = 8.6 μM. Conditions were as in Fig. 3. (b) The spectra of the first principal component derived from the experiments with the mixtures of 1-PB with P450eryF-MDCC taken at a molar ratio of 1:3.3 (dashed line) and 1:1 (solid line).