FIGURE 4.
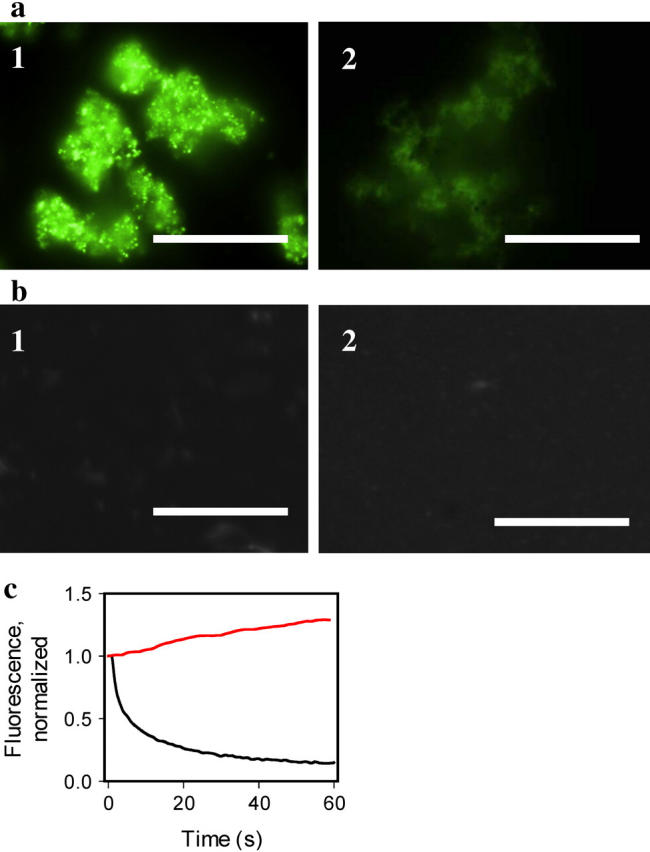
Staining of the amyloid fibrils with hybrid Ag-ThT nanoclusters. (a) Epifluorescence microscopy images of the amyloid fibrils of PrP 90–231 stained with preformed hybrid nanoclusters (panel 1, exposure time for collecting image is 0.02 s), and with 10 μM ThT (panel 2, exposure time 1.6 s), scale bars = 10 μm. Hybrid nanoclusters were generated by irradiation of aqueous solutions of ThT (10 μM)/AgNO3 (1 μM) at 312 nm for 3 min. The amyloid fibrils were diluted to the final concentration of PrP 90–231 equivalent to 1 μM placed on a coverslip and incubated at room temperature in the presence of hybrid nanoclusters or ThT alone. (b) Epifluorescence microscopy of the nonamyloid β-oligomeric form of PrP 90–230 (panel 1, exposure time 1.6 s), and aggregated form of PrP 23–230 (panel 2, exposure time 1.6 s), stained with hybrid nanoclusters. Recombinant human PrP encompassing residues 90–231 was converted in vitro into the amyloid fibrils and the β-oligomeric form as described previously (6). The aggregated form of rPrP 23–230 was prepared as described by Zahn (21) and confirmed by size-exclusion chromatography and dynamic light scattering. (c) Photobleaching kinetics of the fibrils stained with ThT (black line) versus photoactivation kinetics of the fibrils stained with hybrid nanoclusters (red line). The kinetics of photobleaching/photoactivation was collected from a 5 μm × 5 μm area and normalized to the intensity measured at zero time.
