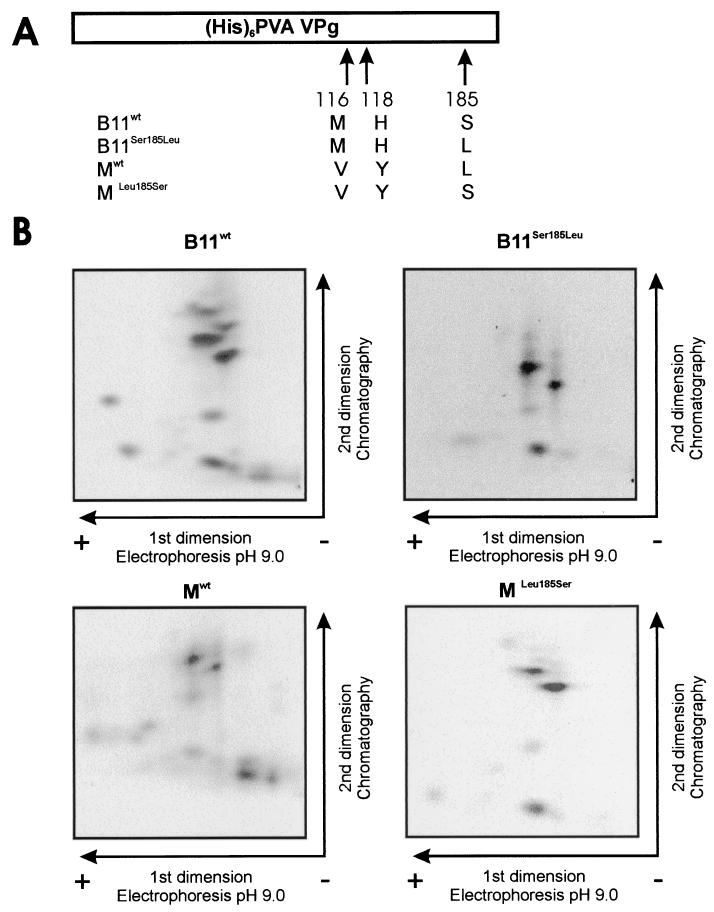
FIG. 6.
Similar phosphorylation patterns obtained for the VPgs of PVA strains B11 and B11-M by using kinase activity from tobacco. (A) Schematic map of the recombinant VPg molecules. The amino acid differences between VPgs B11wt, Mwt, B11Ser185Leu, and MLeu185Ser and the positions of the changed amino acids in VPg are indicated. (His)6, six-His tag. (B) Recombinant VPgs B11wt, Mwt, B11Ser185Leu, and MLeu185Ser were phosphorylated in the presence of 5 mM Mn 2+ in vitro with a total protein extract of tobacco as the kinase source. Phosphorylated proteins were purified by SDS-PAGE, blotted onto nylon membrane, and visualized with Ponceau S. VPg was digested on the membrane with trypsin, and the released peptides were lyophilized and separated on TLE/TLC plates. Radioactive 33P-labeled peptides were autoradiographically visualized.