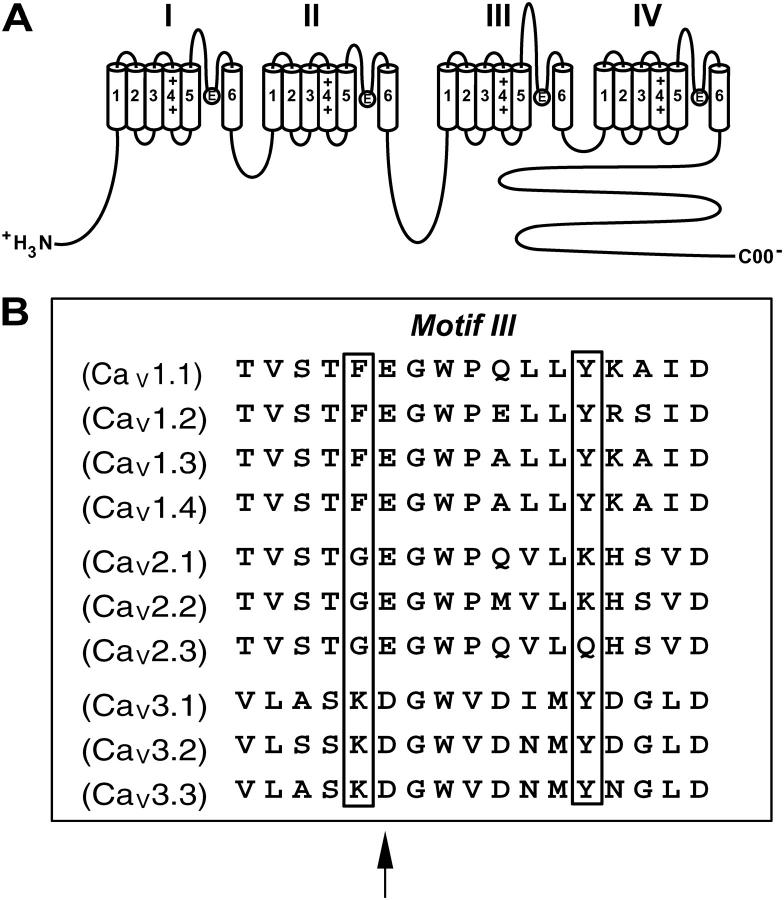
FIGURE 1.
Phe-1144 and Tyr-1152 reside in the pore segment of domain III. (A) Transmembrane folding model of the α1 subunit of high voltage-gated Ca2+ channels. The α1 subunit consists of four homologous domains, each consisting of six transmembrane segments (S1–S6). Four glutamate residues, one residing in each of the four loops connecting S5 and S6 in each domain, form the selectivity filter, or EEEE locus. The selectivity filter is thought to bind a single divalent cation with a high affinity or multiple divalent cations with a low affinity. (B) Alignment of the domain III pore segment from each of the 10 Ca2+ channel family members. All high voltage-activated Ca2+ channels have a glutamate residue at position 1145, and all low voltage-activated Ca2+ channels have an aspartate residue at this position (arrow). All L-type Ca2+ channels (CaV1.1–4) have a phenylalanine, all high voltage-activated non-L-type Ca2+ channels (CaV2.1–3) have a glycine, and all low voltage-activated T-type Ca2+ channels (CaV3.1–3) have a lysine residue at position 1144 (boxed). All L-type Ca2+ channels (CaV1.1–4) and T-type Ca2+ channels (CaV3.1–3) have a tyrosine residue at position 1152, whereas two of three non-L-type Ca2+ channels (CaV2.1–2) have a lysine residue at this position.