Abstract
Antibacterial properties of the frog-derived peptide dermaseptin S4 and a series of synthetic derivatives against the food pathogen Escherichia coli O157:H7 were investigated under extreme incubation conditions. The 28-mer analog K4K20S4 (P28) displayed an MIC of 8 μM and rapid bactericidal kinetics under standard culture conditions. Potent bactericidal properties were maintained at high salt concentrations, under acidic or basic conditions, and at extreme temperatures. The N-terminal 14-mer sequence (P14) displayed higher potency (MIC, 4 μM) but only within a narrow range of incubation conditions, pointing to the importance of the C-terminal domain of P28. The potency range was reextended upon conjugation of aminododecanoic acid to P14. The resulting lipopeptide was even more potent (MIC, 2 μM) and affected bacterial viability under most of the conditions tested, including in commercial apple juice. The mechanistic implications of peptides' hydrophobicity, charge, structure, and binding to an idealized membrane were probed and are discussed here. Collectively, the data indicate interest in simple peptide-based compounds for design of antimicrobials that affect pathogens under a variable range of incubation conditions.
Antimicrobial peptides (AMPs) are important components of innate immunity (23, 39, 52). Many are active towards a wide range of microorganisms by a mode of action which is still not fully understood but is assumed to involve interaction with the bacterial membrane and its disruption. AMPs do not require interaction with a chiral center for activity, supporting a lower probability for microorganisms to develop efficient resistance mechanisms compared with conventional antibiotics (22, 38).
AMPs from the dermaseptin family were recently proposed as model peptides for investigating the effects of acyl conjugation (13, 17, 44). These amphibian-derived AMPs (35, 36) have been amply investigated during the past decade and shown to exert rapid cytolytic activity against a wide range of microorganisms, including gram-negative and gram-positive bacteria, protozoa, filamentous fungi (14, 26, 35, 36), spores of pathogenic bacteria (29), yeasts (11), and intracellular parasites (13, 17, 30), as well as antiviral activity (5).
Due to its distinctive primary structure, dermaseptin S4 was used to identify structure-function relationships, which eventually led to potent derivatives (19, 21, 31, 37, 38). In recent work, we defined the activity of a single-amino-acid-substituted derivative, K4-S4, against Escherichia coli O157:H7 in terms of milieu dependencies (51). Extending that study, the present work is aimed at understanding the molecular elements in native dermaseptin S4 that are necessary for maintaining antimicrobial potency under extreme incubation conditions. We produced a set of derivatives that varied in length, composition, hydrophobicity, and net charge and investigated the effect of incubation conditions on the peptides' activity and bacterial susceptibility. In addition, we investigated the peptides' organization in solution and binding to model membranes.
MATERIALS AND METHODS
Peptides.
Peptides were synthesized by the solid-phase method using 9-fluorenylmethyloxycarbonyl active ester chemistry on a 433A peptide synthesizer (Applied Biosystems) as described previously (19). Acylated analogs were prepared manually by linking the peptide's N terminus to lauric or aminolauric acid as described previously (17). After cleavage from the resin, the crude peptides were purified to 98 to >99% chromatographic homogeneity by reverse-phase high-pressure liquid chromatography (Alliance-Waters). Product identity was confirmed by subjecting the purified peptides to electrospray mass spectrometry (ZQ-Waters). Peptides were stored as a lyophilized powder at 20°C. Prior to experimentation, fresh solutions were prepared in distilled water (1 mg/ml), briefly vortexed, and sonicated, and these were used as stock solutions in all experiments.
Antibacterial assays. (i) Standard incubation conditions.
Bacteria (Escherichia coli O157:H7, ATCC 43895) were grown overnight in LB medium (0.5% NaCl, 0.5% yeast extract, 1% tryptone, pH 7), diluted to 2 × 107 to 4 × 107 CFU/ml in LB, and incubated at room temperature for 60 min prior to being assayed. The stock culture of the strain was maintained in a 50:50 glycerol-LB broth at −80°C.
(ii) MIC determination.
The MIC was determined using the microdilution assay, which was performed in sterilized 96-well plates in a final volume of 200 μl as follows. A stock solution of the peptides was diluted 10-fold in culture medium. One hundred microliters of LB containing bacteria (2 × 107 to 4 × 107 cells/ml) was added to 100 μl LB containing peptide (serial twofold dilutions). Growth was determined by optical density measurements at 620 nm. The MIC was considered the lowest peptide concentration that showed no increase in optical density after overnight incubation at 37°C.
(iii) Bactericidal kinetics.
Unless otherwise stated, the assay was performed in test tubes in a final volume of 1.1 ml. One hundred microliters of bacterial suspension was added to 1 ml of LB containing no peptide or various peptide concentrations. After 0, 5, 15, 30, 60, and 120 min of exposure to peptide at 37°C, cultures were subjected to serial 10-fold dilutions (up to 1/106) by adding 50 μl of sample to 450 μl saline (0.85% NaCl) and kept on ice. Cell counts were determined using the drop plate method (three 20-μl drops onto LB agar plates). CFU were counted after 16 to 24 h of incubation at 37°C.
(iv) Nonstandard incubation conditions.
To assess the effect of temperature variations, assays were performed as described above but bacteria and test tubes containing the culture medium were first incubated for 60 min at the specified temperatures (i.e., 4, 25, 37 and 42°C). For pH and salt variations, the culture medium was brought to the desired pH by adding NaOH or HCl (1 N) or to the desired saline concentration by adding NaCl to the LB.
(v) Apple juice.
Concentrated apple juice (Cider Hagalil; Israel) was diluted by adding 1 ml juice to 6.25 ml double-distilled water (final pH 3.6) and used as a medium as described above. To assess a peptide's availability in apple juice, the peptide was preincubated in apple juice at the concentration of 24 μM (2 h) and then diluted to the desired concentration in LB. The medium was monitored for pH changes throughout.
Liposome preparation.
Small unilamellar vesicles composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (PC-PG) (3:1 molar ratio) were prepared by the sonication method (according to instructions from Avanti Polar) in the buffer corresponding to the incubation conditions used in the antimicrobial assays. The vesicles had a mean diameter of 20 nm as determined by dynamic light scattering. The liposome solution was used as a stock solution for the circular dichroism (CD) and surface plasmon resonance (SPR) experiments.
Peptide organization in solution.
Peptide secondary structure was investigated by CD basically as described previously (31). Peptide (100 μM) was dissolved in a buffer mimicking the medium used for antimicrobial assays (phosphate-buffered saline [PBS] containing 1 to 6% NaCl, acetate buffer [pH 3.6 to 5.5], Tris-HCl buffer [pH 8.5], and PBS preheated or precooled to the appropriate temperatures) in the presence of liposomes (peptide/lipid ratio, 1:20). Three scans and two independent preparations for each type of sample were measured, averaged, and corrected for the contribution of lipid vesicles and buffer.
Peptide self-assembly (aggregation) in buffers was investigated by static light-scattering measurements as described previously (31).
SPR.
Peptide binding to phospholipid membranes was determined using an optical biosensor system (BIAcore 2000, Uppsala, Sweden) based on the principles of surface plasmon resonance (28). The sensor chip L1 (a carboxymethyldextran hydrogel derivatized with lipophilic alkyl chain anchors) was used to prepare a lipid bilayer as described previously (21).
RESULTS
Listed in Table 1 are the dermaseptin S4 analogs that were investigated. Design of the analogs was partly inspired from results obtained in a previous study where dermaseptin S4 and some of the derivatives were evaluated against a nonpathogenic strain of E. coli (19). To find the shortest derivative that is active against the pathogenic strain E. coli O157:H7, the C-terminal domain of S4 was gradually truncated (this group includes three new analogs, i.e., the amide form of K4K20-S4 and the 14- and 12-mer derivatives).
TABLE 1.
Peptides investigated and their properties
| Peptide | Designation | Sequencea | Acetonitrileb (%) | Qc | Mean MIC ± SDd (μM) |
|---|---|---|---|---|---|
| S4 | ALWMTLLKKVLKAAAKAALNAVLVGANA | 69 | 4 | >32 | |
| K4K20S4ae | P28 | ---K---------------K--------NH2 | 60 | 7 | 8 ± 0 |
| K4S4(1-16)a | ---K------------NH2 | 49 | 6 | 4 ± 0 | |
| K4S4(1-15)a | ---K-----------NH2 | 51 | 5 | 4 ± 0 | |
| K4S4(1-14)a | P14 | ---K----------NH2 | 50 | 5 | 4 ± 0 |
| K4S4(1-13)a | ---K---------NH2 | 47 | 5 | 32 ± 0 | |
| K4S4(1-12)a | ---K--------NH2 | 47 | 5 | 32 ± 0 | |
| K4S4(1-10)a | ---K------NH2 | 40 | 4 | >32 | |
| Lauroyl-P14 | C12-P14 | CH3−(CH2)10−CONH−P14 | 74 | 4 | >32 |
| Aminolauroyl-P14 | NC12-P14 | NH2−(CH2)11−CONH−P14 | 55 | 5 | 2 ± 0 |
Primary structures of eight peptides in one-letter code (dashes represent residues identical to those in S4).
Hydrophobicity measure determined by reversed phase high-pressure liquid chromatography (percent acetonitrile/water eluent).
Electrical charge at physiological pH.
Minimal peptide concentration that caused 100% growth inhibition of 107 CFU of E. coli O157 after 24 h of incubation at 37°C in LB medium. Values represent the means and standard deviations obtained from three independent experiments performed in duplicates.
a, amide.
Initial screen: MIC experiments.
As shown in Table 1, the highly hydrophobic native dermaseptin S4 was unable to reach an MIC at the highest concentration tested (MIC, >32 μM), very likely because of its inability to effectively cross the external membrane and reach the cytoplasmic membrane due to its high level of self-assembly in solution (critical micelle concentration = 0.2 μM), as reported previously (44). Therefore, with increasing charge, hydrophobicity was reduced, aggregation was limited, and activity was enhanced (MIC of P28, 8 μM). Truncating the peptide down to the N-terminal 10 residues led to gradually decreased hydrophobicity and charge, which initially correlated with the increased activity (MIC reduced from 8 to 4 μM) observed for the 16- to 14-residue derivatives of comparable hydrophobicity. Thereafter, additional truncations from 13 to 10 residues led to a gradual loss of potency.
Overall, these results showed consistency with published literature as to the importance of charge and hydrophobicity of cationic AMPs and identified a new 14-mer derivative, P14, that was both more economical and more potent than the full-length native peptide or its derivatives.
Bactericidal kinetic assays.
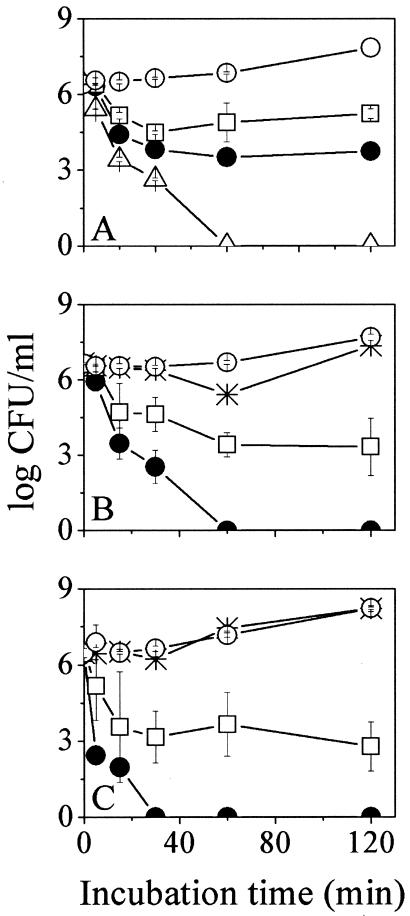
To evaluate the consequences of the C-terminal domain deletion, P28 and P14 were compared in terms of bactericidal kinetics. The dose-response curves obtained for three concentrations of P28 and P14 are shown in Fig. 1A and B, respectively. The time-kill curves show that under equimolar conditions, P14 displayed faster kinetics. For instance, at 8 μM both peptides were able to reduce the bacterial population to half its initial concentration within 30 min; P14, however, eliminated the whole population within 1 h of incubation.
FIG. 1.
Bactericidal kinetics of P28 (A), P14 (B), and NC12-P14 (C) against E. coli O157:H7. Bacteria were incubated at 37°C in the presence of various peptide concentrations (0, 2, 4, 8, and 16 μM, represented by the open circles, asterisks, rectangles, closed circles, and triangles, respectively) in LB medium. Plotted are mean CFU values ± standard deviations obtained from two independent experiments performed in duplicates. Zero CFU indicates negative cultures.
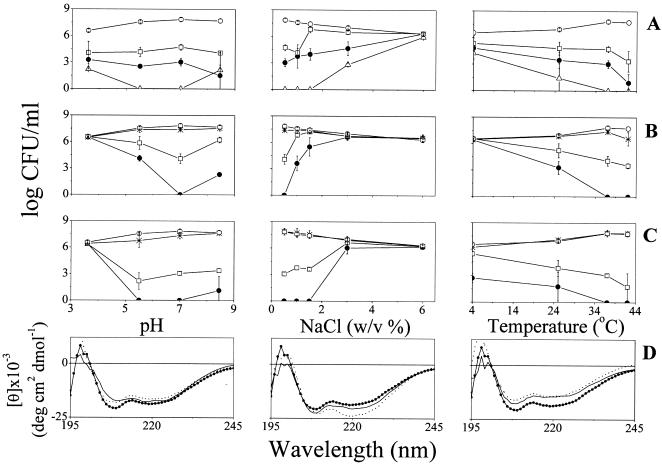
Standard versus extreme incubation conditions.
The bactericidal potencies of P28 and P14 were compared under varied incubation conditions, as summarized in Fig. 2A and B, respectively. Varying the pH from 3.6 to 8.5 hardly affected activity of P28, which displayed a consistent dose response except at high concentration (16 μM), where some loss of potency was observed at the extreme pH values (possibly reflecting peptide aggregation). Unlike P28, P14 displayed potency loss under almost all incubation conditions except standard conditions.
FIG. 2.
Effects of pH, salt, and temperature on E. coli O157:H7 viability and peptide structure. (A to C) Dose responses obtained after 2 h of incubation with P28 (A), P14 (B), and NC12-P14 (C). Symbols and peptide concentrations are as specified for Fig. 1. CFU values represent the means ± standard deviations obtained from two independent experiments performed in duplicates. Zero CFU indicates negative cultures. (D) Effect of incubation conditions on NC12-P14 secondary structure. Circular dichroism spectra were measured for peptide samples (100 μM) in buffer containing liposomes (2 mM PC-PG [3:1]). Data represent average values from two separate experiments.
Effects of peptide acylation.
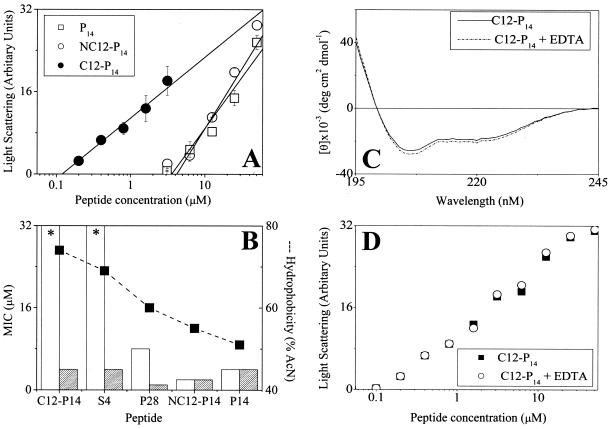
N acylation was recently shown to stabilize structure and improve antimicrobial properties of short dermaseptin derivatives (44) and other AMPs (3, 4, 9, 10, 33, 34, 49). As shown in Table 1, C12-P14 was unable to reach an MIC at the highest concentration tested (MIC, >32 μM), very likely because of its high level of self-assembly in solution (reminiscent of native dermaseptin S4). The fact that acylation also leads to loss of a positive charge is likely to contribute to activity loss. Conjugation of aminolauryl simultaneously restored the original charge and increased hydrophobicity of P14 to a lesser extent than lauryl conjugation. This led to numerous consequences: NC12-P14 displayed an MIC of 2 μM (Table 1) and faster kinetics both under standard conditions (Fig. 1C) and most of the other incubation conditions tested (Fig. 2C).
Peptide organization in solution.
Self-assembly states in PBS are shown in Fig. 3A for P14 and its acylated derivatives. C12-P14 was found to assemble at low concentrations (critical micelle concentration estimated at ∼0.1 μM), whereas NC12-P14 behaved similarly to P14, which is assumed to be monomeric. This supports the hypothesis that the inactivity of C12-P14 against gram-negative bacteria is linked to its aggregated state. To further explore this possibility, antibacterial activity was compared in presence of EDTA, which is known to induce defects in the outer membrane and increase its permeability. Hydrophobic peptides displayed potency only in presence of EDTA. EDTA did not affect the MICs of P14 and NC12-P14 (Fig. 3B). Similarly, EDTA did not affect peptide organization in solution, as verified using circular dichroism (Fig. 3C) or aggregation properties (Fig. 3D). These results link inactivity with aggregation and indicate that the aminoacyl contributed hydrophobicity while avoiding aggregation.
FIG. 3.
Peptide organization in solution and consequences for antibacterial activity. (A) Peptide self-assembly in PBS as investigated by static light-scattering measurements. The intensity of scattered light was plotted against total peptide concentrations, and linear regression analysis was performed on the data at the concentration range close to the monomer-micelle transition zone. The critical micelle concentration was evaluated by extrapolating the curve to the intercept with the x axis. (B) Activities of various derivatives against E. coli O157, determined in LB medium in the absence (white bars) or presence (hatched bars) of 20 mM EDTA. The derivatives are organized according to decreasing hydrophobicity. Asterisks indicate that an MIC was not observed at the highest concentration tested (32 μM). Values in panels A and B represent the means ± standard deviations from two independent experiments performed in duplicate. The lack of an error bar indicates low or no standard deviation. Hydrophobicity (dashed line) and MIC (histogram) were determined as described in the footnotes to Table 1. (C) Circular dichroism spectra of the designated peptides as described for Fig. 2 in the presence and absence of EDTA. (D) Effect of EDTA on peptide self-assembly in PBS as described for panel A.
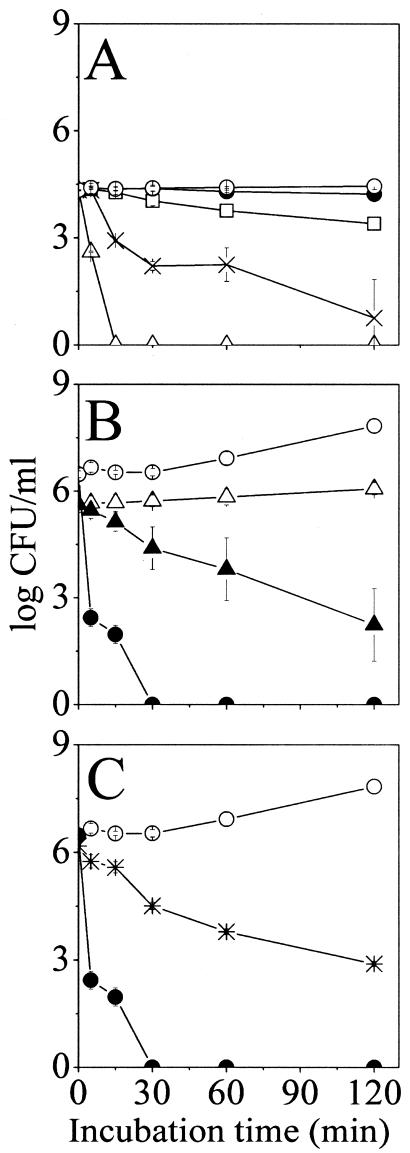
Bactericidal properties in apple juice.
Consistent with the results obtained under incubation at low pH in LB medium (Fig. 2), P28, P14, and NC12-P14 displayed reduced potency in apple juice (not shown). Figure 4A shows the dose-dependent time-kill curves obtained for NC12-P14 as an example. To account for the differences in activity at acidic conditions, NC12-P14 was assayed using two experimental variations designed to distinguish between causes related to bacteria versus peptide: (i) bacteria were preincubated at pH 3.6 and then assayed at neutral pH, and (ii) the peptide was preincubated in apple juice prior to being assayed in LB medium at pH 7 as described above. The results are summarized in Fig. 4B and C, respectively. Bacteria that were preincubated at an acidic pH were less susceptible to NC12-P14. In fact, even in the absence of peptide, bacterial viability was hampered, pointing to a possible stress response adaptation of bacteria being the cause for reduced potency. Moreover, peptide preincubation in apple juice also led to reduced potency (Fig. 4C), pointing to peptide inactivation by a juice component(s). Furthermore, a lower bactericidal effect was observed when apple juice was brought to pH 7 by adding NaOH (not shown).
FIG. 4.
Bactericidal kinetics of NC12-P14 in apple juice and effects of preincubations. (A) Bacteria were incubated in the absence of NC12-P14 (open circles) and in presence of 8, 16, 32, and 64 μM NC12-P14 (closed circles, rectangles, asterisks, and triangles, respectively) in commercial apple juice (pH 3.6). CFU counts were performed after the specified incubation periods at 37°C. (B) Bacteria were incubated overnight in LB medium either at pH 3.6 (triangles) or pH 7 (circles), diluted to 106 cells/ml in LB medium at pH 7, and incubated for 2 hours in the absence (open symbols) or presence (closed symbols) of 8 μM NC12-P14. CFU counts were performed after the specified incubation periods at 37°C. (C) The peptide was incubated for 2 hours in commercial apple juice, diluted to 8 μM in LB medium, and incubated for 2 hours with bacteria. The graph describes bacterial viability in the absence of peptide (open circles) and in the presence of preincubated (asterisks) and nonpreincubated (closed circles) peptide.
Secondary-structure considerations.
CD measurements were performed for P28, P14, or NC12-P14 in buffers containing liposomes that mimic bacterial membranes. All three peptides displayed typical spectra with unordered structures in buffer (not shown), but in the presence of liposomes, CD profiles indicated a clear shift toward a typical α-helix, as characterized by double minima at 208 and 220 nm. Figure 2D summarizes the CD data obtained for NC12-P14 as a representative peptide. The spectra suggest that the helical conformation is essentially maintained irrespective of the conditions tested. Compared with standard conditions, both extreme pH and temperature conditions might somewhat reduce but certainly not eliminate the helical conformation. Similarly, NaCl somewhat increased helicity, as reported for other antimicrobial peptides (27, 50); note, however, that even under conditions that displayed the highest helicity (e.g., 6% NaCl), peptides were inactive, and vice versa. As inactivity could not be correlated to evident loss of structure, we conclude that activity might require structure but that structure does not necessarily indicate activity.
Binding experiments.
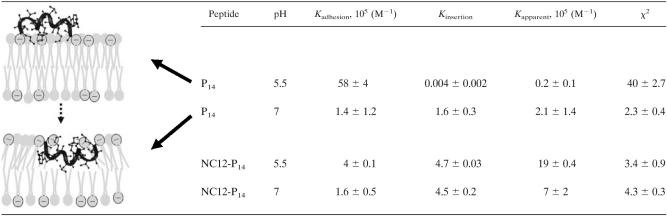
Binding properties of P14 and NC12-P14 were compared at pH 7 and 5.5, as summarized in Table 2. Other peptides and conditions could not be evaluated due to the aggregation state and membrane instability at extreme pH values.
TABLE 2.
Effect of pH on properties of peptide binding to a bilayer membranea
The cartoon illustrates our interpretation of the binding events according to the two-step binding model. Adhesion to a negatively charged surface (upper drawing) is followed by insertion (lower drawing) into the membrane hydrophobic core. The table summarizes SPR data obtained using a PC-PG (3:1) membrane. Values represent the means and standard deviations obtained from three independent experiments performed in duplicates. χ2 is indicative of the level of fitting of the binding model to the measured data and is lowest for optimal fit.
At neutral pH, the SPR outcome was consistent with the antibacterial assays: the initial interactions of the peptides were similar as indicated by identical adhesion affinity and a tendency to insert into the membrane, although with a 2.8-fold difference in insertion affinities, which correlated with their observed antibacterial properties in terms of either MIC or bactericidal effect. At acidic pH, however, they differed at both stages of interaction. Although both peptides displayed higher adhesion affinities, P14 showed nearly 15-fold-higher affinity. Remarkably, P14 had a much lower propensity to insert at acidic pH, and consequently, the overall binding constant was nearly 100-fold lower, which again correlated with antibacterial properties (Fig. 2). It is interesting to note that high adhesion affinity (at acidic pH) could be what that prevents P14 from going forward with the reaction and inserting into the membrane.
Since P14 displayed a low insertion affinity, its binding constants should in fact be calculated using a simple bimolecular association model (Langmuir kinetics); the association affinity constant of this reaction is calculated as the ratio of association to dissociation rate and has the same meaning as Kadhession in the two-stage reaction model. Indeed, the affinity constants calculated according to the two models were rather similar (25 × 105 versus 58 × 105 M−1), but the chi square value of the bimolecular model was much lower (4.6 versus 40), indicating a better fit. These considerations support the assumption that P14 does not insert into the lipid bilayer at acidic pH.
DISCUSSION
This study focused on understanding the structural and physical properties affecting bactericidal potency under various conditions and showed how sequence manipulations led to potent derivatives. In this respect, several comparisons seem of interest, particularly in distinguishing between reasons for reduced potency. Comparing P14 and NC12-P14 suggests that increased hydrophobicity improved activity (as previously documented). It is interesting to note, therefore, that various short derivatives displayed increased potency relative to P28, despite reduced hydrophobicity and charge. Furthermore, raising the hydrophobicity beyond a certain limit decreases activity, as seen with C12-P14. We speculate, based on the combined results from light-scattering and MIC experiments, that beyond the repulsion exerted by the hydrophilic outer membrane on hydrophobic compounds (as observed for hydrophobic penicillins, for instance), AMPs that aggregate in solution lose their ability to cross the outer membrane and to affect the inner membrane (9, 19, 31). Figure 3 shows this correlation and further demonstrates that in presence of EDTA, i.e., under conditions where the outer membrane is unstable, aggregated AMPs become active. We hypothesize, based on these observations, that activity emerged because the EDTA-mediated destabilization of the outer membrane facilitated passage of aggregated AMPs which were then able to interact with the inner (plasma) membrane. The data in Table 1 also support this hypothesis. NC12-P14 is more active despite being more hydrophobic than most derivatives (which furthermore display similar or even higher positive charge). It becomes inactive only upon aggregation (C12 analog). We estimate that beyond a hydrophobicity level roughly corresponding to 60% eluent, chances are that potency against gram-negative bacteria will be low, although this limit is to be established for individual compounds. This obstacle may be overcome by specific strategies (as in the present study).
Another type of obstacle was encountered when incubation conditions were altered. Although short derivatives displayed improved bactericidal activity, P14 did not maintain potency under acidic conditions or at high salt concentrations or low temperatures. What are the reasons for this reduced efficiency? The virulent form of E. coli designated serotype O157:H7 has highly efficient mechanisms of global stress resistance, which contribute to its low infectious dose and tolerance to stress factors, including acidic pH (40). The alternative sigma factor (σ2) encoded by rpoS which regulates expression of various stress response genes (32, 42, 43) was implicated in stress resistance of Salmonella and Escherichia (15, 16, 18, 20, 47). These stress responses may reduce bacterial susceptibility to antimicrobial peptides by either membrane modifications, SOS gene expression, or changing the transmembrane potential. Our data with preincubated bacteria under acidic conditions support this hypothesis. Also, the E. coli transmembrane potential reportedly decreases significantly as a stress response to acidic conditions (46); this could explain the reduced propensity for insertion as evidenced by SPR experiments. Accordingly, pH was stipulated to affect the mode of action by which clavanin A permeabilizes the Lactobacillus sake membrane (48).
Collectively, the present data point to potential uses of dermaseptin derivatives and like compounds in various antimicrobial fields, particularly in food safety. Antimicrobial properties of several AMPs were studied under various incubation conditions relevant to food products. The lactic acid bacterium-derived nisin is the only AMP used today by the food industry (24), despite several disadvantages. Low solubility at physiological pH reduces the activity of nisin and limits its use (45). Also, nisin is inefficient against yeasts, molds, and gram-negative bacteria unless other processing technologies are used in combination, such as addition of chelator agents (8, 12), preheating (7), and pH reduction (45). Among the animal-derived AMPs studied, a magainin analog displayed an MIC range of 3 to 50 μg/ml, with reduced potency at 4 and 25°C (1). Similarly, a 14-residue model peptide, 6K8L (6, 25), which reduced the E. coli population in citrate buffer by 3.5 log units after 10 min of incubation at 5 μg/ml (2), induced the same effect in apple juice only after 8 h incubation at 100 μg/ml. Likewise, another synthetic bactericidal peptide, [RLLR]5, displayed an 8- to 32-fold decrease in antibacterial and antifungal potencies in the presence of 200 mM NaCl (41).
In conclusion, this study points to three elements that affect peptide activity under extreme incubation conditions: peptide-membrane interactions (interplay between adhesion and insertion affinities), the bacterial stress response (which is irrelevant to the peptide's properties), and peptide availability (captivating interactions with food components). In addition, this study identified a potent and economical new derivative, NC12-P, which was shown to maintain activity under incubation conditions that represent those used for many food products, and therefore its design may be useful in developing design strategies for antimicrobial compounds that are able to affect pathogen viability under a large spectrum of incubation conditions.
Acknowledgments
This research was supported by the Israel Science Foundation (grant 387/03).
REFERENCES
- 1.Abler, L. A., A. Klapes, B. W. Sheldon, and T. R. Klaenhammer. 1995. Inactivation of food-borne pathogens with magainin peptides. J. Food Prot. 58:381-388. [DOI] [PubMed] [Google Scholar]
- 2.Appendini, P., and J. H. Hotchkiss. 2000. Antimicrobial activity of a 14-residue synthetic peptide against foodborne microorganisms. J. Food Prot. 63:889-893. [DOI] [PubMed] [Google Scholar]
- 3.Avrahami, D., and Y. Shai. 2002. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry 41:2254-2263. [DOI] [PubMed] [Google Scholar]
- 4.Avrahami, D., and Y. Shai. 2004. A new group of antifungal and antibacterial lipopeptides derived from non-membrane active peptides conjugated to palmitic acid. J. Biol. Chem. 279:12277-12285. [DOI] [PubMed] [Google Scholar]
- 5.Belaid, A., M. Aouni, R. Khelifa, A. Trabelsi, M. Jemmali, and K. Hani. 2002. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66:229-234. [DOI] [PubMed] [Google Scholar]
- 6.Blondelle, S. E., and R. A. Houghten. 1992. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry 31:12688-12694. [DOI] [PubMed] [Google Scholar]
- 7.Boziaris, I. S., L. Humpheson, and M. R. Adams. 1998. Effect of nisin on heat injury and inactivation of Salmonella enteritidis PT4. Int. J. Food Microbiol. 43:7-13. [DOI] [PubMed] [Google Scholar]
- 8.Branen, J. K., and P. M. Davidson. 2004. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 90:63-74. [DOI] [PubMed] [Google Scholar]
- 9.Chicharro, C., C. Granata, R. Lozano, D. Andreu, and L. Rivas. 2001. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 45:2441-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu-Kung, A. F., K. N. Bozzelli, N. A. Lockwood, J. R. Haseman, K. H. Mayo, and M. V. Tirrell. 2004. Promotion of peptide antimicrobial activity by fatty acid conjugation. Bioconjug. Chem. 15:530-535. [DOI] [PubMed] [Google Scholar]
- 11.Coote, P. J., C. D. Holyoak, D. Bracey, D. P. Ferdinando, and J. A. Pearce. 1998. Inhibitory action of a truncated derivative of the amphibian skin peptide dermaseptin s3 on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 42:2160-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutter, C. N., and G. R. Siragusa. 1995. Efficacy of organic acids against Escherichia coli O157:H7 attached to beef carcass tissue using a pilot scale model carcass washer. J. Food Prot. 57:97-103. [DOI] [PubMed] [Google Scholar]
- 13.Dagan, A., L. Efron, L. Gaidukov, A. Mor, and H. Ginsburg. 2002. In vitro antiplasmodium effects of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 46:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Lucca, A. J., J. M. Bland, T. J. Jacks, C. Grimm, and T. J. Walsh. 1998. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med. Mycol. 36:291-298. [PubMed] [Google Scholar]
- 15.Diez-Gonzalez, F., and J. B. Russell. 1999. Factors affecting the extreme acid-resistance of Escherichia coli O157:H7. Food Microbiol. 16:367-374. [Google Scholar]
- 16.Dodd, C. E., and T. G. Aldsworth. 2002. The importance of RpoS in the survival of bacteria through food processing. Int. J. Food Microbiol. 74:189-194. [DOI] [PubMed] [Google Scholar]
- 17.Efron, L., A. Dagan, L. Gaidukov, H. Ginsburg, and A. Mor. 2002. Direct interaction of dermaseptin S4 aminoheptanoyl derivative with intraerythrocytic malaria parasite leading to increased specific antiparasitic activity in culture. J. Biol. Chem. 277:24067-24072. [DOI] [PubMed] [Google Scholar]
- 18.Fang, F. C., C. Y. Chen, D. G. Guiney, and Y. Xu. 1996. Identification of sigma S-regulated genes in Salmonella typhimurium: complementary regulatory interactions between sigma S and cyclic AMP receptor protein. J. Bacteriol. 178:5112-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feder, R., A. Dagan, and A. Mor. 2000. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 20.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 21.Gaidukov, L., A. Fish, and A. Mor. 2003. Analysis of membrane-binding properties of dermaseptin analogues: relationships between binding and cytotoxicity. Biochemistry 42:12866-12874. [DOI] [PubMed] [Google Scholar]
- 22.Ge, Y., D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler, and M. Zasloff. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 24.Hansen, J. N. 1994. Nisin as a model food preservative. Crit. Rev. Food Sci. Nutr. 34:69-93. [DOI] [PubMed] [Google Scholar]
- 25.Haynie, S. L., G. A. Crum, and B. A. Doele. 1995. Antimicrobial activities of amphiphilic peptides covalently bonded to a water-insoluble resin. Antimicrob. Agents Chemother. 39:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez, C., A. Mor, F. Dagger, P. Nicolas, A. Hernandez, E. L. Benedetti, and I. Dunia. 1992. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 59:414-424. [PubMed] [Google Scholar]
- 27.Johansson, J., G. H. Gudmundsson, M. E. Rottenberg, K. D. Berndt, and B. Agerberth. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718-3724. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson, U., L. Fagerstam, B. Ivarsson, B. Johnsson, R. Karlsson, K. Lundh, S. Lofas, B. Persson, H. Roos, I. Ronnberg, S. Sjulander, E. Stenberg, R. Stahlberg, C. Urbaniczky, H. Ostlin, and M. Malmqvist. 1991. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques 11:620-627. [PubMed] [Google Scholar]
- 29.Jouenne, T., A. Mor, H. Bonato, and G. A. Junter. 1998. Antibacterial activity of synthetic dermaseptins against growing and non-growing Escherichia coli cultures. J. Antimicrob. Chemother. 42:87-90. [DOI] [PubMed] [Google Scholar]
- 30.Krugliak, M., R. Feder, V. Y. Zolotarev, L. Gaidukov, A. Dagan, H. Ginsburg, and A. Mor. 2000. Antimalarial activities of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 44:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kustanovich, I., D. E. Shalev, M. Mikhlin, L. Gaidukov, and A. Mor. 2002. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. J. Biol. Chem. 277:16941-16951. [DOI] [PubMed] [Google Scholar]
- 32.Lin, J., M. P. Smith, K. C. Chapin, H. S. Baik, G. N. Bennett, and J. W. Foster. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockwood, N. A., J. R. Haseman, M. V. Tirrell, and K. H. Mayo. 2004. Acylation of SC4 dodecapeptide increases bactericidal potency against Gram-positive bacteria, including drug-resistant strains. Biochem. J. 378:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majerle, A., J. Kidric, and R. Jerala. 2003. Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J. Antimicrob. Chemother. 51:1159-1165. [DOI] [PubMed] [Google Scholar]
- 35.Mor, A., V. H. Nguyen, A. Delfour, D. Migliore-Samour, and P. Nicolas. 1991. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 30:8824-8830. [DOI] [PubMed] [Google Scholar]
- 36.Mor, A., and P. Nicolas. 1994. Isolation and structure of novel defensive peptides from frog skin. Eur. J. Biochem. 219:145-154. [DOI] [PubMed] [Google Scholar]
- 37.Mor, A., and P. Nicolas. 1994. The NH2-terminal alpha-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 269:1934-1939. [PubMed] [Google Scholar]
- 38.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277-304. [DOI] [PubMed] [Google Scholar]
- 40.Padhye, N. V., and M. P. Doyle. 1992. Escherichia coli O157:H7: epidemiology, pathogenesis, and methods for detection in food. J. Food Prot. 55:555-565. [DOI] [PubMed] [Google Scholar]
- 41.Park, I. Y., J. H. Cho, K. S. Kim, Y.-B. Kim, M. S. Kim, and S. C. Kim. 2004. Helix stability confers salt resistance upon helical antimicrobial peptides. J. Biol. Chem. 279:13896-13901. [DOI] [PubMed] [Google Scholar]
- 42.Price, S. B., C. M. Cheng, C. W. Kaspar, J. C. Wright, F. J. DeGraves, T. A. Penfound, M. P. Castanie-Cornet, and J. W. Foster. 2000. Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microbiol. 66:632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price, S. B., J. C. Wright, F. J. DeGraves, M. P. Castanie-Cornet, and J. W. Foster. 2004. Acid resistance systems required for survival of Escherichia coli O157:H7 in the bovine gastrointestinal tract and in apple cider are different. Appl. Environ. Microbiol. 70:4792-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radzishevsky, I. S., S. Rotem, F. Zaknoon, L. Gaidukov, A. Dagan, and A. Mor. 2005. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob. Agents Chemother. 49:2412-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayman, K., N. Malik, and A. Hurst. 1983. Failure of nisin to inhibit outgrowth of Clostridium botulinum in a model cured meat system. Appl. Environ. Microbiol. 46:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richard, H., and J. W. Foster. 2004. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 186:6032-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner, K., J. Porter, R. Pickup, and C. Edwards. 2000. Changes in viability and macromolecular content of long-term batch cultures of Salmonella typhimurium measured by flow cytometry. J. Appl. Microbiol. 89:90-99. [DOI] [PubMed] [Google Scholar]
- 48.van Kan, E. J., R. A. Demel, E. Breukink, B. A. van der Bent, and B. de Kruijff. 2002. Clavanin permeabilizes target membranes via two distinctly different pH-dependent mechanisms. Biochemistry 41:7529-7539. [DOI] [PubMed] [Google Scholar]
- 49.Wakabayashi, H., H. Matsumoto, K. Hashimoto, S. Teraguchi, M. Takase, and H. Hayasawa. 1999. N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob. Agents Chemother. 43:1267-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcox, W., and D. Eisenberg. 1992. Thermodynamics of melittin tetramerization determined by circular dichroism and implications for protein folding. Protein Sci. 1:641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yaron, S., T. Rydlo, D. Shachar, and A. Mor. 2003. Activity of dermaseptin K-4-S4 against foodborne pathogens. Peptides 24:1815-1821. [DOI] [PubMed] [Google Scholar]
- 52.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]