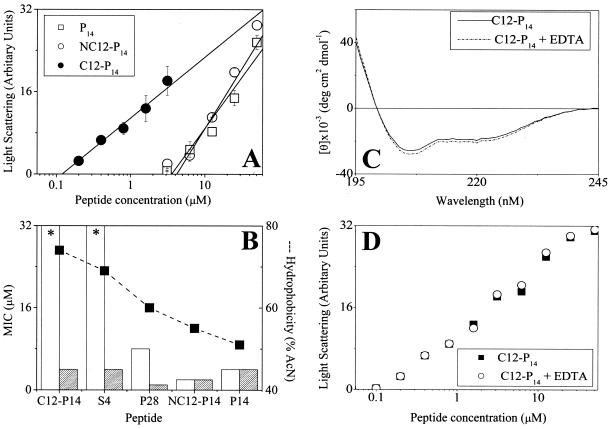
FIG. 3.
Peptide organization in solution and consequences for antibacterial activity. (A) Peptide self-assembly in PBS as investigated by static light-scattering measurements. The intensity of scattered light was plotted against total peptide concentrations, and linear regression analysis was performed on the data at the concentration range close to the monomer-micelle transition zone. The critical micelle concentration was evaluated by extrapolating the curve to the intercept with the x axis. (B) Activities of various derivatives against E. coli O157, determined in LB medium in the absence (white bars) or presence (hatched bars) of 20 mM EDTA. The derivatives are organized according to decreasing hydrophobicity. Asterisks indicate that an MIC was not observed at the highest concentration tested (32 μM). Values in panels A and B represent the means ± standard deviations from two independent experiments performed in duplicate. The lack of an error bar indicates low or no standard deviation. Hydrophobicity (dashed line) and MIC (histogram) were determined as described in the footnotes to Table 1. (C) Circular dichroism spectra of the designated peptides as described for Fig. 2 in the presence and absence of EDTA. (D) Effect of EDTA on peptide self-assembly in PBS as described for panel A.