Abstract
In fluid monolayers approaching collapse, phospholipids and their complexes with diacylglycerols hinder adsorption to the monolayer of the amphipathic protein, colipase. Herein, a statistical, free-area model, analogous to that used to analyze two-dimensional lipid diffusion, is developed to describe regulation by lipids of the initial rate of protein adsorption from the bulk aqueous phase to the lipid-water interface. It is successfully applied to rate data for colipase adsorption to phospholipid alone and yields realistic values of the two model parameters; the phospholipid excluded area and the critical free surface area required to initiate adsorption. The model is further developed and applied to analyze colipase adsorption rates to mixed monolayers of phospholipid and phospholipid-diacylglycerol complexes. The results are consistent with complexes being stably associated over the physiologically relevant range of lipid packing densities and being randomly distributed with uncomplexed phospholipid molecules. Thus, complexes should form in fluid regions of cellular membranes at sites of diacylglycerol generation. If so, by analogy with the behavior of colipase, increasing diacylglycerol may not trigger translocation of some amphipathic peripheral proteins until its abundance locally exceeds its mole fraction in complexes with membrane phospholipids.
INTRODUCTION
Lipid-mediated signaling in cells is part of the chain of events through which chemical information from outside the cell causes changes in gene expression in the nucleus. An important site along such signaling pathways is the plasma membrane. Basically, on the cell surface the interaction of a soluble ligand with a transmembrane receptor can trigger the metabolism of phospholipids on the inner surface of the bilayer membrane to generate lipid second messengers, like diacylglycerols (1). These, in turn, trigger the adsorption to the membrane of proteins residing in the cell cytoplasm, known as peripheral proteins, thereby initiating signaling cascades that ultimately terminate in the nucleus. Peripheral proteins that are regulated by diacylglycerols, e.g., protein kinase C, typically possess specific domains, like C1 and C2 domains, that help target them to the membrane. For example, GPF-tagged C1 domains alone will partition to cell membranes in response to diacylglycerol generation (2). To accomplish this, such protein domains typically have solvent-exposed hydrophobic residues on loops or other surfaces (3).
Mechanistically, it is unclear how changes in lipid composition in a membrane trigger peripheral protein adsorption. For example, C1 domains have a hydrophilic cleft surrounded by hydrophobic residues in which phorbol esters or short-chain diacylglycerols bind. This creates a large hydrophobic surface (4), the dehydration of which ultimately drives the partitioning of the domain to the membrane. However, naturally occurring diacylglycerols generated in membranes are relatively apolar compared to phospholipids and, in contrast to phorbol esters and short-chain diacylglycerols, exhibit negligible water solubility (5). This raises the kinetic question of how do membrane-resident diacylglycerol and cytoplasmic protein efficiently find each other (6), i.e., what regulates the initial translocation of peripheral proteins to the membrane?
In some cases protein-membrane interactions are mediated by specific interactions of the peripheral protein with specific motifs of membrane-resident proteins (7) and in others by electrostatic interactions with the exposed charges of anionic membrane lipids (8). Another contributing mechanism, suggested by a growing body of in vitro evidence (9), is that the localized enzymatic generation of diacylglycerols in the membrane creates transient diacylglycerol clusters that are relatively dehydrated and act as sites for the initial interaction of amphipathic peripheral proteins with the interface. This model is based largely on measurements of the regulation of the initial rate of binding of a small, single-domain amphipathic protein, colipase, to monomolecular films composed of phospholipid and lipid molecules lacking a phosphoryl-X headgroup, like diacylglycerols, monoacylglycerols, and fatty acids. Mathematically, the dependence of the initial rate on diacylglycerol composition at high total lipid packing density has been reasonably well described by a statistical model in which 1), each phospholipid molecule is assumed to behave as a site that prevents colipase adsorption; and 2), colipase adsorption is initiated by clusters of sites occupied by diacylglycerol that exceed a critical size.
Comparison of phospholipid-excluded areas determined in the presence and absence of diacylglycerol invariably gave larger values when diacylglycerol was present (9). This was puzzling because diacylglycerol alone does not lower colipase adsorption rate. However, there was independent evidence that diacylglycerol and phospholipid could form complexes, suggesting that the complexes, like phospholipid alone, were inhibitory. Supporting this idea were 1), the observed absence of significant colipase adsorption rate at high lipid packing densities and diacylglycerol mole fractions below that of the complexes; and 2), an almost 1:1 correlation between excluded areas of different phospholipids and the apparent molecular areas of those phospholipids in the complexes or alone at monolayer collapse pressure. Importantly, the phospholipid excluded areas were determined from colipase adsorption rates to mixed lipid monolayers with no a priori assumption of complex formation, while the areas of the phospholipid alone and in complexes were determined from physical characterization of the mixed monolayers in the absence of colipase.
In the previous studies on which the above model is based, all measurements of colipase adsorption rates to mixed-lipid monolayers were made in the liquid-expanded (fluid) state at high lipid packing densities that equal or exceed the range found in cell membranes. For the monomolecular films used, this means surface pressures close to monolayer collapse. Moreover, the composition-dependence of the surface pressure of monolayers at collapse, as interpreted in light of the two-dimensional phase rule, is also the basis for demonstrating the existence of complexes in the absence of colipase. This raises the question of whether phospholipid-diacylglycerol complexes are a limiting state determined by the mutual fit of differently shaped, weakly-interacting molecules confined to a small area or whether they persist at lower lipid densities. To address this we have measured colipase adsorption rates as a function of lipid packing density in mixed monolayers containing diacylglycerol in a range of compositions over which it should remain complexed with phospholipid if complexes arise from an energetically stable packing arrangement.
To analyze the data, we have developed and implemented an improved statistical model for calculating the availability of potential colipase adsorption sites. This model is first applied to experimental data obtained with a diacylphosphatidylcholine and then to its mixtures with a diacylglycerol in the range of diacylglycerol mole fractions over which it should be complexed, 0–0.3. The results show that the model describes all data sets well and that complexes persist at and below packing densities representative of those found in biological membranes.
MATERIALS AND METHODS
Experimental methods
The phospholipid, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was from Avanti Polar Lipids (Alabaster, AL) and the diacylglycerol, 1,2-dioleoylglycerol (DO) was from Sigma (St. Louis, MO). The purification of water and preparation of solvents, buffer, and lipid solutions have been previously described in Momsen et al. (10). Porcine colipase used in this study was prepared and converted to [14C]colipase by reductive methylation as described previously in Schmit et al. (11). The [14C]colipase had a specific radioactivity of 9.76 Ci/mol.
Experimental details for measuring the rate of adsorption of [14C]colipase to lipid monolayers have been described previously in Mizuno et al. (12). Briefly, a cylindrical Teflon trough (surface area 20.4 cm2, volume 19.5 ml) was filled with phosphate-buffered saline held at 24°C. Lipid films were spread from a hexane/ethanol (95:5) solution until the desired surface pressure was reached. After allowing the lipid monolayer to stabilize for 5 min, stirring (50 rpm) was started and, after 2 min, colipase solution was injected through a port in the side of the trough to achieve 22 nM in the aqueous phase. Stirring was continued for 10 min, after which the monolayer was collected on one side of a hydrophobic filter-paper disk. The amount of colipase adsorbed to the monolayer was determined by liquid scintillation counting. Based on prior studies, 90% recovery of the monolayer and 15 μl of carryover of subphase onto the paper were assumed (13).
Surface pressure-molecular area isotherms of the lipid mixtures used were determined with an automated Langmuir film balance using protocols that have been recently described in detail (14). The isotherms provided the lipid packing density corresponding to the initial surface pressure of each colipase adsorption rate measurement.
Model
We developed a statistical model of protein adsorption to lipid monolayers. The following assumptions of the model are based on our previous experimental and theoretical results (9):
Above a certain lipid packing density, colipase does not adsorb to phospholipid monolayers. The area per phospholipid below which colipase cannot adsorb (the excluded area) is APL.
Above a certain lipid packing density, colipase does not adsorb to dynamic complexes of phospholipids and diacylglycerols. The area per molecule below which colipase cannot adsorb to dynamic complexes (the excluded area) is Acplx.
Surface area that is not occupied by excluded areas of phospholipids and dynamic complexes (free area) is potentially available for colipase adsorption. Adsorption is initiated at those regions of the monolayer where the fraction of the free area is larger than a threshold value. For successful adsorption of a colipase molecule of cross-sectional area, AE, the free area should be equal to or larger than the critical area, Acr.
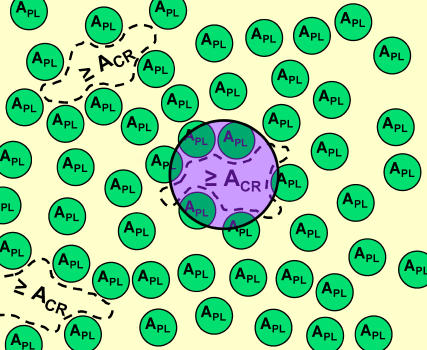
These model concepts for colipase adsorption to a one-component phospholipid monolayer are shown pictorially in Fig. 1, and frequently used symbols are defined in Appendix 1.
FIGURE 1.
Free-area model for the rate-determining step of protein adsorption to a phospholipid monolayer. Smaller green circles (APL) represent a snapshot of the distribution of excluded areas of randomly-arranged phospholipid molecules and the purple circle represents the area of a protein molecule as it approaches the monolayer from the adjacent aqueous phase. Protein adsorption can occur only in free areas greater than or equal to a critical free area, Acr, as indicated by the dashed lines.
One-component monolayer
In the case of a one-component phospholipid (PL) monolayer, it is the excluded surface area of the phospholipid where colipase does not adsorb, while it potentially adsorbs to the rest of the surface (free surface). More precisely, it is assumed that the colipase, of cross-sectional area, AE, initially interacts only with free areas of the monolayer above a critical value, Acr. Once fully adsorbed, colipase inserts into the monolayer and occupies an area, AE, among the PL molecules.
The free area at a phospholipid molecule is fluctuating around a thermodynamic average. According to the thoroughly tested theory of lipid lateral diffusion in one-component lipid bilayers (15–17) and monolayers (18), the free area per lipid molecule follows a Poisson distribution. Thus, the probability that the free area around a phospholipid is (a, a + da),
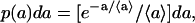 |
(1) |
where 〈a〉 is the average free area per lipid molecule. Let us consider now k lipid molecules of the monolayer, where the respective free areas per molecule are: a1, a2, a3, …, ak, and the total free area belonging to these molecules is a1 + a2 + a3 + … + ak−1 < b ≤ a1 + a2 + a3 + … + ak. The probability that the total free area of k molecule is not smaller than b is
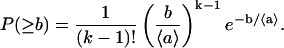 |
(2) |
This is a Poisson distribution. The derivation of Eq. 2, given in Appendix 2, assumes the lateral homogeneity of the monolayer. This assumption is valid for a one-component one-state lipid monolayer, such as the observed POPC monolayer in liquid-expanded state.
When colipase approaches a one-component phospholipid monolayer, it covers part of the surface area, AE. The excluded surface area of the phospholipid molecules that are completely or partially in this monolayer region is k′ APL, where the number of molecules, k′, is a real number. The free area belonging to these k′ molecules cannot be smaller than b′, where
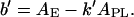 |
(3) |
By using Eqs. 2 and 3, the probability that the free area of k′ molecules located in the considered surface region is not smaller than b′ is
 |
(4) |
where k′(b′) = [AE = b′]/APL. In Eq. 4 the continuous version of the discrete factorial function, k!, the γ-function, Γ(k), is utilized because k′ is a real number. By a further substitution, x = b′/〈a〉, we get
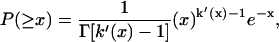 |
(5) |
where k′(x) = [AE = x〈a〉]/APL. Colipase is able to adsorb to and occupy a region of the monolayer surface of area AE, when it collides with a free area larger than the critical value, Acr. The probability that the free area, in a region of surface area AE, is larger than Acr, i.e., the probability of colipase initial adsorption is
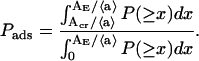 |
(6) |
The initial adsorption rate of the colipase is measured at different lateral pressures, which correspond to different areas per lipid, Aexp. The initial adsorption rate (normalized to the maximal initial adsorption rate) can be compared with the calculated adsorption probability, Pads, i.e., one can fit Eq. 6 to the experimental data. There are two model parameters in Eq. 6, APL and Acr. In Eq. 6, the free area per PL, 〈a〉, can be obtained from the measured area per PL, Aexp, as 〈a〉 = Aexp − APL. Note that Pads is a conditional probability with the condition that 0 ≤ b′ ≤ AE.
Two-component monolayer
The normalized adsorption rate of colipase has been measured for two-component monolayers, containing NDG diacylglycerol and NPL phospholipid molecules. From independently measured surface pressure-area isotherms, the monolayer average area per molecule, Aexp, is known at each surface pressure and lipid composition.
As long as the diacylglycerol-phospholipid dynamic complexes are randomly distributed in the monolayer, one can calculate the normalized adsorption rate by using again Eq. 6. This is the case because, in this range of diacylglycerol mole fractions, there are two populations of free areas: the free areas of phospholipid molecules that do not form complexes with diacylglycerol molecules, and the free areas of the rest of the molecules. Both of these populations have a Poisson distribution with different Poisson parameters. The distribution of the sum of these two free areas is also a Poisson distribution (see Appendix 3).
In the case of a two-component monolayer the free area per molecule 〈a〉 in Eq. 6 can be obtained from the measured specific area, Aexp, as 〈a〉 = Aexp − (Aexcl/N), where Aexcl is the total excluded area of the monolayer surface where colipase cannot adsorb and N = NDG + NPL is the total number of diacylglycerol and phospholipid molecules. The model parameters in Eq. 6 are Acr and Aexcl/N.
RESULTS
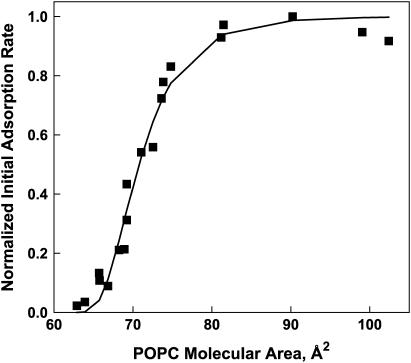
In Fig. 2, square symbols mark the normalized initial adsorption rates measured at different specific areas for one-component POPC monolayers. Normalization was accomplished by dividing the measured rates by the highest value measured. The data are qualitatively of sigmoid shape. Calculated values were obtained by fitting Eq. 6 to the experimental points and these are connected by solid line segments. There is good agreement between the measured and calculated values. The values of the model parameters, APL and Acr, obtained from the fitting procedure, are 58.3 and 84.8 Å2, respectively, and the sum of the squares of the deviations between measured and calculated rates is 0.066.
FIGURE 2.
Colipase adsorption to one-component phospholipid (POPC) monolayers. The normalized initial adsorption rate of colipase is plotted against the specific area of POPC molecules forming a monolayer at the water-air interface. (Solid squares show experimental data; line segments join fitted values determined using Eq. 6.)
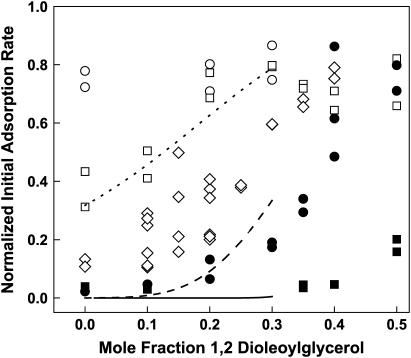
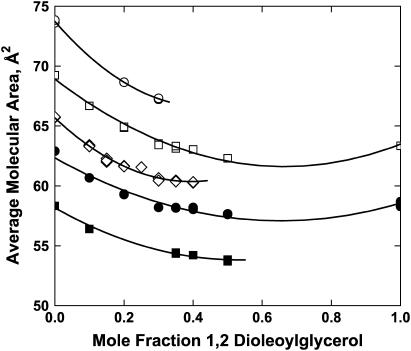
Colipase adsorption to lipid monolayers was also measured in the case of two-component, DO/POPC monolayers. In Fig. 3, the measured normalized initial adsorption rate of colipase is plotted against the DO mole fraction, X. Adsorption rates obtained at different lateral pressures are marked by different symbols. Solid symbols show that, within the range of physiologically relevant surface pressures (19,20), adsorption is largely prevented. Overall, the figure shows that the adsorption rate tends to increase with increasing DO mole fraction and/or decreasing lateral pressure. Note that obtaining the rate data at specific values of the surface pressure is not required for subsequent analysis but merely facilitates experimentation and the global analysis of the data (below). The area per lipid molecule corresponding to each point shown is known from the experimental surface pressure-area isotherm measured independently for each lipid composition (Fig. 4). In Fig. 4, the area per molecule is plotted against the DO mole fraction at the experimental lateral pressures. The figure shows that the experimental specific areas over which the adsorption rate varied from zero to maximal ranged from 55 to 75 Å2/molecule. At each lateral pressure a second-order polynomial, ao + a1X + a2X2, is fitted to the area/mol fraction data (Fig. 4, solid lines; see also Discussion, below). The polynomial fitting parameters are given in Table 1.
FIGURE 3.
Colipase adsorption to two-component (POPC/DO) monolayers. The normalized initial adsorption rates of colipase obtained at initial surface pressures of 15 (○), 20 (□), 25 (⋄), 30 (•), and 40 (▪) mN/m are plotted against the DO mole fraction. Representative continuous fit lines through data obtained at 20 (dotted), 30 (dashed), and 40 (solid) mN/m up to Xcplx were generated by substituting Eq. 7 into Eq. 6 with the fitted values of APL = 58.3 Å2, Acr = 84.8 Å2, and β = −29.5 Å2 and molecular areas generated using the polynomial fitting parameters in Table 1 (see also text in Colipase Adsorption to Two-Component Monolayers at X ≤ Xcplx).
FIGURE 4.
Specific areas of POPC/DO monolayers at selected lateral pressures. Specific areas were determined from surface pressure-area isotherms determined separately for the lipid mixtures in the absence of colipase. Symbols are the same as for Fig. 3. (Lines) A second-order polynomial was fitted to area per molecule data taken at each initial surface pressure and the resulting polynomial parameters are given in Table 1.
TABLE 1.
Parameters of the area per molecule versus DO mole fraction curves
| Lateral pressure (mN/m) | a0 | a1 | a2 | r* |
|---|---|---|---|---|
| 15 | 73.73 ± 0.07 | −33.2 ± 1.3 | 38.8 ± 4.3 | 0.999 |
| 20 | 68.90 ± 0.16 | −22.0 ± 0.8 | 16.6 ± 0.8 | 0.993 |
| 25 | 65.67 ± 0.12 | −27.0 ± 1.3 | 34.4 ± 2.9 | 0.992 |
| 30 | 62.32 ± 0.25 | −16.0 ± 1.1 | 12.3 ± 1.0 | 0.975 |
| 40 | 58.18 ± 0.17 | −16.4 ± 1.7 | 15.5 ± 3.2 | 0.996 |
The value r is the correlation coefficient of the fit.
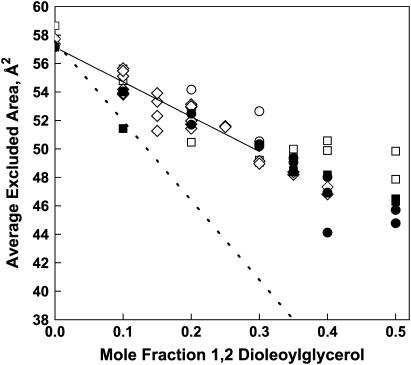
When fitting Eq. 6 to the data in Fig. 3, we take Acr = 84.8 Å2, the value obtained for one-component POPC monolayers. In so doing, we assume that Acr is independent of the DO mole fraction. This assumption is based on our observation that the adsorption rate to the free area of a phospholipid monolayer equals the adsorption rate to a tightly-packed pure DO monolayer (see Fig. 2 in Sugár et al. (21)). Since Acr is assumed to be independent of the DO mole fraction, the observed increase in the colipase adsorption rate with increasing DO should be caused by the dependence of the remaining model parameter, Aexcl/N, on the DO mole fraction. To reveal this dependence we fitted Eq. 6 separately to each data point in Fig. 3. In Fig. 5, the calculated values of Aexcl/N are plotted against the DO mole fraction. There is a definite decrease in the excluded area per molecule from 58 Å2 to 49 Å2 as the DO mole fraction increases from 0 to 0.35. Note that if the only contribution to Aexcl/N was from the phospholipid alone, i.e., it is simply being diluted by DO, the value of Aexcl/N at X = 0.35 would be only 38 Å2 (APL[1 – X] = 58.3 × 0.65 = 38) rather than 49 Å2 (Fig. 5, dotted line). Thus, DO clearly contributes to Aexcl/N in the mixed monolayers. Above a DO mole fraction of X = 0.35, the excluded area per molecule keeps decreasing at lateral pressures: 25, 30, and 40 mN/m, but not at 20 mN/m. Note also that X = 0.35 is greater than but close to the DO mole fraction in the complex composition, Xcplx = 0.3, as determined from the partial phase diagram constructed from surface pressure-area isotherms for DO-POPC lipid mixtures (21).
FIGURE 5.
Calculated excluded area per molecule in POPC/DO monolayers. The calculated excluded area per molecule, Aexcl/N, is plotted against the DO mole fraction. Excluded areas were obtained by fitting Eq. 6 separately to each data point shown in Fig. 3, using APL = 58.8 Å2 and Acr = 84.8 Å2 from the fit of the data in Fig. 2. Symbols are as in Fig. 3. The solid line up to the DO mole fraction in the complex was calculated by using Eq. 7 with the globally fit value of β. The dashed line shows expected behavior of the average excluded area if DO were acting as a simple POPC diluent.
DISCUSSION
With decreasing lateral surface pressure, phospholipid specific area increases. As this occurs, the normalized initial adsorption rate of colipase abruptly increases from zero to almost one, both with phospholipid alone (Fig. 2) and with mixtures containing diacylglycerol (Fig. 3). As specific area increases, segments of the hydrocarbon tails of the phospholipid molecules should become exposed between lipid headgroups, generating hydrophobic, i.e., headgroup-free, area to which amphipathic proteins can adsorb. A statistical model, the so-called free area model (see Model, above), describes this phenomenon in quantitative agreement with the experimental data. There are similarities and differences between this newer model and our older model (21):
The older model was applicable at high surface pressures and a range of compositions (X ≥ Xcplx), over which free area among complexes and uncomplexed diacylglycerol is negligible, and no uncomplexed phospholipid is present. The newer model is applicable in a range of surface pressures and compositions (X ≤ Xcplx), over which free area among complexes and uncomplexed phospholipid molecules is significant, and no uncomplexed diacylglycerol is present.
In both models, the protein can adsorb to free areas exceeding a critical value, Acr; however, the physical representation of the free area is different in the two models. In the older model, it is the surface occupied by uncomplexed diacylglycerol molecules, whereas in the newer model it is the area between the excluded areas of both complexes and uncomplexed phospholipid molecules.
In the older model, the size of the clusters of uncomplexed diacylglycerol molecules follows a binomial distribution, whereas, in the newer model, the free areas follow a Poisson distribution. According to the newer model, colipase adsorption may occur only at those places in the monolayer surface where the free area exceeds a critical value, rather than a critical number of discrete sites as in the previous model.
Colipase adsorption to one-component phospholipid monolayers
The increase of colipase adsorption rate as POPC packing density is decreased is well described by the free-area model using two adjustable parameters, the excluded area of POPC, APL = 58.3 Å2, and the critical area required for colipase adsorption, Acr = 84.8 Å2. The value of the former is consistent with the observed absence of colipase adsorption at that specific area (Fig. 2) and approximates the molecular area of POPC at monolayer collapse (14). This implies that the hydrated polar heads of the lipid molecules dominate the monolayer surface and preclude colipase adsorption. Consistent with this interpretation, the area of co-planar, minimally hydrated phosphorylcholine headgroups, estimated from phosphatidylcholine crystal structure data, is 56.8 Å2 (22)—an area similar to APL of POPC.
The value of Acr = 84.8 Å2 is ≪AE = 500 Å2, the monolayer surface area occupied by a fully adsorbed colipase molecule (10). This is consistent with the assumptions of the model and emphasizes that Acr is the critical area necessary to initiate the adsorption process (Fig. 1). It will be of interest to determine critical areas for other amphipathic proteins/domains of known structure to determine how Acr varies with the extent of exposed hydrophobic surface. Potentially, a protein with a smaller Acr would begin to adsorb at lower free areas than one with a larger Acr, even though the latter may ultimately be more tightly adsorbed.
Colipase adsorption to two-component monolayers at X ≤ Xcplx
Our previous conclusions about the formation of lipid complexes and their effects on colipase adsorption were based mostly on monolayer physical properties and colipase adsorption rates measured at surface pressures near monolayer collapse, which occurs at ∼47 mN/m (23). Although those studies were consistent with complexes being stable entities, the limited range of packing densities used did not allow differentiation between stable complexes and fortuitous packing arrangements. Using colipase adsorption rates as a monitor of excluded area, we have herein investigated the stability of DO-POPC complexes over a range of surface pressures, 15–40 mN/m, and at low diacylglycerol mole fractions, X ≤ Xcplx. Our model-based analysis of this data is consistent with DO-POPC complexes being stable molecular associations within a range of surface pressures that includes the physiologically relevant pressure range of 30–35 mN/m (19,20).
The driving force for complex formation may be favorable interactions between lipid species that increase with lipid packing density or unfavorable interactions between the uncomplexed species and water or free area at low packing densities. It should be noted that the term complex, when used to describe interactions in monolayers, does not necessarily imply an isolatable species (24). Rather, in two dimensions it may also refer to a preferred packing arrangement of the form XnpYnq, where p/(p+q) gives the mole fraction of X at the complex composition, Xcplx. The determination of n is problematic and, for small n, complexes can be miscible with excess X or Y (25). In fluid monolayers, like the DO-POPC monolayers used here, miscibility between uncomplexed diacylglycerol or phospholipid and their complex (12) is routinely observed.
Whereas the ability of uncomplexed POPC to inhibit colipase adsorption is characterized by APL, the ability of DO-POPC complexes to inhibit colipase adsorption is characterized by the excluded area per lipid molecule in the complex, Aexcl/N. The value of this parameter decreases with increasing DO mole fraction over the range 0.0–0.35 DO and is independent of lateral pressure at each composition (Fig. 5). The linear decrease of the excluded area per molecule with increasing DO mole fraction is consistent with both stoichiometric complex formation and the random lateral distribution of the complex and POPC assumed by the free-area model. The negative slope in Fig. 5 signifies that, in a complex, DO is less efficient in inhibiting colipase adsorption than is POPC on a per-molecule basis, despite the molecular area of DO alone being slightly larger than that of POPC (26). Importantly, the loss of excluded surface per molecule with increasing DO cannot be explained by simple dilution of POPC; DO clearly contributes to the inhibition, but is simply less efficient than POPC. This observation is consistent with the umbrella model of lipid complex formation (27) in that DO may be partially shielded by the phosphorylcholine headgroup.
As noted above, with an increasing number of independent, randomly distributed dynamic complexes, the number of condensed regions of the monolayer surface linearly increases and the average excluded area per molecule linearly decreases. Thus (see Appendix 4), one can derive a formula for the slope of the Aexcl/N versus X line where NPL/z > NDG,
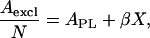 |
(7) |
and where β = [(1 + z)(Acplx – APL)], with Acplx being the excluded area per molecule of a dynamic complex. According to this equation, in Fig. 5 the slope of the solid line, β, is equal to the difference between the excluded area of a dynamic complex and the excluded area of (z+1) uncomplexed phospholipid molecules.
The value of β can be determined by global optimization. Equation 7 provides a relationship between the excluded area per molecule and the DO mole fraction,  After substituting Eq. 7 into Eq. 6 we get a general equation for colipase adsorption to phospholipid-diacylglycerol two-component monolayers. The general equation contains three model parameters: APL, Acr, and β. The values of the first two model parameters are available from the fit to adsorption rate data taken at zero DO composition. Using these two values we performed a global fit of the general equation to all the DO/POPC data at X ≤ Xcplx. With the general equation and the model parameter values one can calculate the initial adsorption rates only at those DO compositions at which the monolayer area per molecule was measured. If we want to calculate the initial adsorption rate at any DO composition, one has to know the area per molecule as a function of the composition at each experimental lateral pressure. For this we used the parameters given in Table 1 and generated continuous lines, as exemplified in Fig. 3. From the global optimization the best fit was with β = −29.5 Å2. Interestingly, the fitted value of |β| is one-half of the specific area of DO alone at monolayer collapse (26) and also one-half of the excluded area of POPC (Table 1). Whether these relationships are coincidental or have significance will require studies involving diacylglycerol and phospholipid species with different molecular areas.
After substituting Eq. 7 into Eq. 6 we get a general equation for colipase adsorption to phospholipid-diacylglycerol two-component monolayers. The general equation contains three model parameters: APL, Acr, and β. The values of the first two model parameters are available from the fit to adsorption rate data taken at zero DO composition. Using these two values we performed a global fit of the general equation to all the DO/POPC data at X ≤ Xcplx. With the general equation and the model parameter values one can calculate the initial adsorption rates only at those DO compositions at which the monolayer area per molecule was measured. If we want to calculate the initial adsorption rate at any DO composition, one has to know the area per molecule as a function of the composition at each experimental lateral pressure. For this we used the parameters given in Table 1 and generated continuous lines, as exemplified in Fig. 3. From the global optimization the best fit was with β = −29.5 Å2. Interestingly, the fitted value of |β| is one-half of the specific area of DO alone at monolayer collapse (26) and also one-half of the excluded area of POPC (Table 1). Whether these relationships are coincidental or have significance will require studies involving diacylglycerol and phospholipid species with different molecular areas.
Using β, the ∼2:1 ratio of POPC to DO in the complex and the value of APL, the area of a complex unit, POPC2DO1, is (1 + z)Acplx = (1 + z)APL + β ≈ 146 Å2. Expressed per molecule of POPC in the complex, this is 73 Å2. This value is similar to the values of 76 and 79 Å2 obtained earlier by applying the site-based analytical model to rate data obtained by compositional variation above the complex composition at high lipid packing density with the stearoyl homolog of POPC (21). Thus, within the limits of comparison, the ability of complexes to inhibit colipase adsorption appears to be independent of whether the free area surrounding them is generated by changing their packing density (Fig. 3) or by the presence of uncomplexed DO molecules in excess of the complex composition (21).
Colipase is a single domain protein that was used in this and previous studies as a model peripheral protein. These studies show that the inhibition of colipase adsorption by phospholipids and their complexes with diacylglycerol can be overcome in vitro either by lowering total lipid packing density or by increasing the mole fraction of diacylglycerol sufficiently above Xcplx. For peripheral proteins involved in cell-signaling mechanisms, however, initiation of adsorption by the former condition is unavailable because, globally, phospholipid-packing density in membranes must remain approximately constant. This is because macroscopic changes of more than a few percent in lipid-specific area, i.e., surface pressure changes of 3–10 mN/m depending on loading rate, result in rupture (28,29), and because the coefficient of thermal expansion of phospholipids is <10−3/°K (15). Rather, what may transiently change phospholipid-packing density locally in cell membranes is the conversion of phospholipids to diacylglycerols as a consequence of the action of phospholipases C. For the mole fraction of diacylglycerols to locally exceed Xcplx the rate of diacylglycerol generation must be either faster than the lateral equilibration of their concentration by diffusion or diacylglycerols must be laterally confined, or both. With respect to confinement, there is convincing evidence that cellular plasma membranes are subdivided by the cytoskeleton into zones with sizes of 30–200 nm (≈1000–50,000 phospholipid molecules) in which lipids reside for periods of 1–17 ms before hopping to another zone (30). Thus, it is likely that high, transient mole fractions of diacylglycerols are generated in the vicinity of phospholipase C molecules after cell stimulation, triggering the translocation of some peripheral proteins to the membrane without compromising global membrane integrity.
CONCLUSION
A free-area model is developed and used to describe the rate-determining step of protein adsorption from the aqueous phase to lipid interfaces. The application of this model to colipase adsorption at one-component and two-component interfaces demonstrates clearly the stability and inhibitory nature of phospholipid-diacylglycerol complexes. In the lipid compositional range studied here, fractions of area free of phospholipid and complex as large as those used to assess complex stability in this study are nonphysiological because membrane lipids are tightly packed. However, bilayer membrane packing density is below that of monolayer collapse, near which complex formation and its effects on protein translocation have been previously studied. The present results show that in the range of lipid packing density of cell membranes, complexes do not dissociate and should inhibit the adsorption of peripheral protein domains. This supports the notion that, in cell membranes, exceeding the diacylglycerol composition of the complex may be required to trigger adsorption of some peripheral proteins.
Acknowledgments
I.P.S. is grateful for the support of Dr. Diomedes Logothetis and Mrs. Lawrence Garner.
This work was supported by U.S. Public Health Service grant No. HL49180 and the Hormel Foundation.
APPENDIX 1: DEFINITIONS OF FREQUENTLY USED SYMBOLS
N, the total number of lipid molecules in the interface.
NPL, the number of phospholipid molecules in the interface.
NDG, the number of diacylglycerol molecules in the interface.
Aexp, the experimental area per lipid molecule.
APL, the excluded area exhibited by an uncomplexed phospholipid molecule.
Aexcl/N, the average excluded area per lipid molecule.
Acr, the minimum free area required to initiate colipase adsorption.
AE, the area occupied by a fully adsorbed colipase molecule.
X, the mole fraction of diacylglycerol in the interface.
Xcplx, the mole fraction of diacylglycerol in a phospholipid-diacylglycerol complex.
Acplx, the excluded area per molecule in a phospholipid-diacylglycerol complex.
APPENDIX 2: THE PROBABILITY THAT THE FREE AREA OF K LIPID MOLECULES IS NOT SMALLER THAN B
Let us consider k lipid molecules of the monolayer with the following set of free areas: The free area of the first k−1 molecules fall into the intervals (a1,a1 + da1), (a2,a2 + da2), …, and (ak−1,ak−1 + dak−1), whereas the free area of the kth molecule falls into (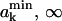 ), where
), where 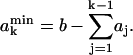 Sets of areas where
Sets of areas where 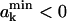 are rejected. This choice of the free areas ensures that the total free area of the considered k molecules is not smaller than b.
are rejected. This choice of the free areas ensures that the total free area of the considered k molecules is not smaller than b.
The probability that the free area of molecule i(≤k − 1) falls into the interval (ai,ai + dai) is
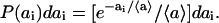 |
The probability that the free area of molecule k falls into the interval (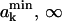 ) is
) is
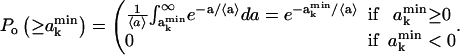 |
The probability that the total free area of the considered k molecules is larger than b can be obtained from the multiple convolution (31)
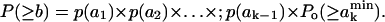 |
To calculate the multiple convolutions, one has to multiply the generating functions of the individual distributions,
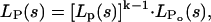 |
where
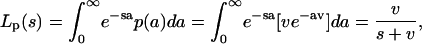 |
and
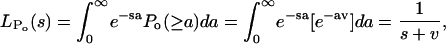 |
where
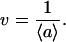 |
The inverse of the generating function Lp(s) results in the probability that the total free area of the considered k molecules is not smaller than b,
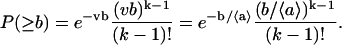 |
This is a Poisson distribution, where b/〈a〉 = 〈k〉 is the average number of molecules with total free area not less than b.
APPENDIX 3: THE CONVOLUTION OF TWO POISSON DISTRIBUTIONS IS A POISSON DISTRIBUTION
Let us consider the following, A and B, Poisson distributions:
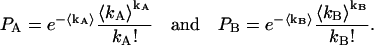 |
The convolution of these distributions, PAB = PA × PB, gives the probability of getting kA and kB so that their sum is given, kAB (= kA + kB). The product of the generating functions of the A and B distribution results in the generating function of the convoluted distribution,
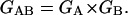 |
The generating function of distribution A is
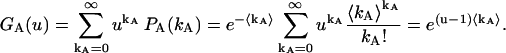 |
and similarly for distribution B is
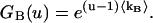 |
Thus the generating function of the convoluted distribution is
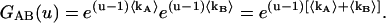 |
We may notice that this is the generating function of the Poisson distribution,
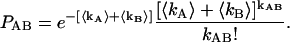 |
APPENDIX 4: THE SIGNIFICANCE OF THE EXCLUDED AREA IN A TWO-COMPONENT SYSTEM
The number of molecules in a two-component monolayer is
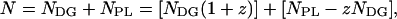 |
where z is the number of phospholipid molecules in a dynamic complex. The total number of molecules forming dynamic complexes is given by the first square bracket and the second square bracket gives the number of uncomplexed phospholipid molecules.
The number of uncomplexed phospholipid molecules cannot be negative, and thus Eq. 7 is valid when
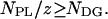 |
Both dynamic complexes and uncomplexed phospholipids contribute to the excluded area of the monolayer, and thus the excluded area per molecule is
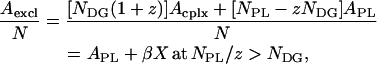 |
where Acplx is the excluded area per molecule of a dynamic complex and
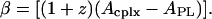 |
References
- 1.Brose, N., A. Betz, and H. Wegmeyer. 2004. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr. Opin. Neurobiol. 14:328–340. [DOI] [PubMed] [Google Scholar]
- 2.Oancea, E., M. N. Teruel, A. F. G. Quest, and T. Meyer. 1998. Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J. Cell Biol. 140:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stahelin, R. V., and W. Cho. 2001. Differential roles of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2. Biochemistry. 40:4672–4678. [DOI] [PubMed] [Google Scholar]
- 4.Zhang, G., M. G. Kazanietz, P. M. Blumberg, and J. H. Hurley. 1995. Crystal structure of the Cys2 activator-binding domain of protein kinase Cδ in complex with phorbol ester. Cell. 81:917–924. [DOI] [PubMed] [Google Scholar]
- 5.Small, D. M. 1970. Surface and bulk interactions of lipids and water with a classification of biologically active lipids based on these interactions. Fed. Proc. 29:1320–1326. [PubMed] [Google Scholar]
- 6.Hurley, J. H., and T. Meyer. 2001. Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 13:146–152. [DOI] [PubMed] [Google Scholar]
- 7.Dorn II, G. W., and D. Mochly-Rosen. 2002. Intracellular transport mechanisms of signal transducers. Annu. Rev. Physiol. 64:407–429. [DOI] [PubMed] [Google Scholar]
- 8.Denisov, G., S. Wanaski, P. Luan, M. Glaser, and S. McLaughlin. 1998. Binding of basic peptides to membranes produces lateral domains enriched in the acidic lipids phosphatidylserine and phosphatidylinositol 4,5-bisphosphate: an electrostatic model and experimental results. Biophys. J. 74:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugar, I. P., N. K. Mizuno, M. M. Momsen, W. E. Momsen, and H. L. Brockman. 2003. Regulation of lipases by lipid-lipid interactions: implications for lipid-mediated signaling in cells. Chem. Phys. Lipids. 122:53–64. [DOI] [PubMed] [Google Scholar]
- 10.Momsen, M. M., M. Dahim, and H. L. Brockman. 1997. Lateral packing of the pancreatic lipase cofactor, colipase, with phosphatidylcholine and substrates. Biochemistry. 36:10073–10081. [DOI] [PubMed] [Google Scholar]
- 11.Schmit, G. D., M. M. Momsen, W. G. Owen, S. Naylor, A. Tomlinson, G. Wu, R. E. Stark, and H. L. Brockman. 1996. The affinities of pro-colipase and colipase for interfaces are regulated by lipids. Biophys. J. 71:3421–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuno, N. K., J. M. Smaby, B. A. Cunningham, M. M. Momsen, and H. L. Brockman. 2003. Phospholipid-diacylglycerol complexes regulate colipase adsorption to monolayers. Langmuir. 19:1802–1808. [Google Scholar]
- 13.Momsen, W. E., and H. L. Brockman. 1997. Recovery of monomolecular films in studies of lipolysis. Methods Enzymol. 286:292–305. [DOI] [PubMed] [Google Scholar]
- 14.Brockman, H. L., K. R. Applegate, M. M. Momsen, W. C. King, and J. A. Glomset. 2003. Packing and electrostatic behavior of sn-2-docosahexaenoyl and -arachidonoyl phosphoglycerides. Biophys. J. 85:2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galla, H.-J., W. Hartmann, U. Theilen, and E. Sackmann. 1979. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J. Membr. Biol. 48:215–236. [DOI] [PubMed] [Google Scholar]
- 16.MacCarthy, J. E., and J. J. Kozak. 1982. Lateral diffusion in fluid systems. J. Chem. Phys. 77:2214–2216. [Google Scholar]
- 17.Vaz, W. L. C., R. M. Clegg, and D. Hallmann. 1985. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 24:781–786. [DOI] [PubMed] [Google Scholar]
- 18.Peters, R., and K. Beck. 1983. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc. Natl. Acad. Sci. USA. 80:7183–7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh, D. 1996. Lateral pressure in membranes. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald, R. C. 1996. The relationship and interactions between lipid bilayers vesicles and lipid monolayers at the air/water interface. In Vesicles. M. Rosoff, editor. Marcel Dekker, New York. 3–48.
- 21.Sugar, I. P., N. K. Mizuno, M. M. Momsen, and H. L. Brockman. 2001. Lipid lateral organization in fluid interfaces controls the rate of colipase association. Biophys. J. 81:3387–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pascher, I., M. Lundmark, P.-G. Nyholm, and S. Sundell. 1992. Crystal structures of membrane lipids. Biochim. Biophys. Acta. 1113:339–373. [DOI] [PubMed] [Google Scholar]
- 23.Lee, S., D. H. Kim, and D. Needham. 2001. Equilibrium and dynamic interfacial tension measurements at microscopic interfaces using a micropipette technique. 2. Dynamics of phospholipid monolayer formation and equilibrium tensions at the water-air interface. Langmuir. 17:5544–5550. [Google Scholar]
- 24.Dervichian, D. G. 1958. The existence and significance of molecular associations in monolayers. In Surface Phenomena in Chemistry and Biology. J.F. Danielli, K.G.A. Pankhurst, and A.C. Riddiford, editors. Pergamon Press, London, UK. 70–87.
- 25.Radhakrishnan, A., and H. M. McConnell. 1999. Condensed complexes of cholesterol and phospholipids. Biophys. J. 77:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smaby, J. M., and H. L. Brockman. 1990. Surface dipole moments of lipids at the argon-water interface. Similarities among glycerol-ester-based lipids. Biophys. J. 58:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, J. 2002. Exploration of molecular interactions in cholesterol superlattices: effect of multibody interactions. Biophys. J. 83:1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olbrich, K., W. Rawicz, D. Needham, and E. Evans. 2000. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 79:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans, E., V. Heinrich, F. Ludwig, and W. Rawicz. 2003. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 85:2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murase, K., T. Fujiwara, Y. Umemura, K. Suzuki, R. Iino, H. Yamashita, M. Saito, H. Murakoshi, K. Ritchie, and A. Kusumi. 2004. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 86:4075–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feller, W. 1966. Probability distribution in Rr. In An Introduction to Probability Theory and Its Applications. W. Feller, editor. John Wiley & Sons, New York. 125–164.