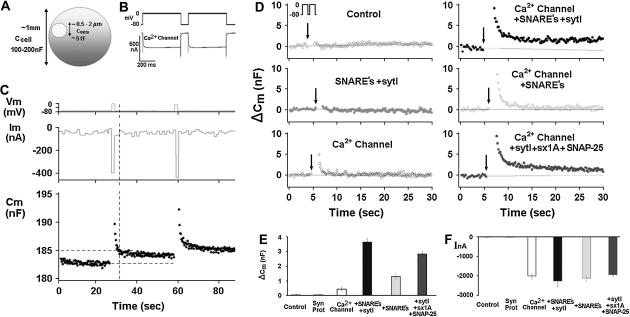
FIGURE 1.
Reconstitution of depolarization-induced exocytosis in oocytes by expression of exogenous proteins. (A) Diameter and capacitance of plasma membrane and cortical granules in Xenopus oocytes. (B) Effect of depolarization on membrane capacitance in a Xenopus oocyte expressing a voltage-gated Ca2+ channel. (Upper trace) Depolarizing voltage command (see Methods), consisting of depolarization from a holding potential of −80 mV to 0 mV for 2 × 500 ms, separated by 100 ms at −80 mV). (C) Continuous monitoring of membrane capacitance showing the effect of depolarization on membrane capacitance (Cm) in an oocyte expressing, Ca2+ channel, Lc-type Ca2+ channel subunits α11.2, β2A, α2δ; SNAREs: syntaxin 1A, SNAP-25, and synaptotagmin; representative original traces of voltage (upper) current (middle) and Cm (lower). The vertical dashed line indicates the time at which Cm was read for comparison with the baseline Cm before depolarization. (D) Monitoring Cm in oocytes expressing different proteins. Control, uninjected oocytes; other panels, oocytes expressing heterologously synaptic proteins: synaptic proteins, syntaxin 1A, SNAP-25, synaptobrevin, and synaptotagmin, Ca2+ channel, Lc-type Ca2+ channel subunits α11.2, β2A, α2δ; SNAREs: syntaxin 1A, SNAP-25 and synaptobrevin; synaptotagmin I, sytI; Ca2+ channel plus syntaxin 1A, SNAP-25 and syt I. (E) Summary: effect of depolarization on Cm. Groups as in D. ΔCm, depolarization-induced change of membrane capacitance; bars show mean ± SD (n = 13). (F) Effect of depolarization on membrane current. Depolarization-induced membrane current (InA; mean ± SD, n = 13 each). Data show that the Cm changes (C) cannot be explained by membrane current, but rather reflect bona fide exocytosis.