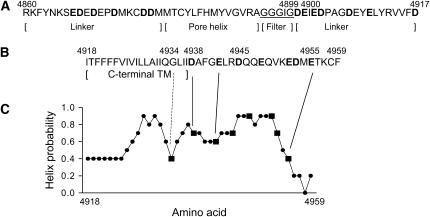
FIGURE 1.
Pore-lining sequences of RyR1. Shown are the amino acid sequences of lumenal loop spanning the two most C-terminal transmembrane-spanning segments (A) and the most C-terminal transmembrane-spanning segment (C-terminal TM) and an extension by 22 residues (B). C-terminal TM corresponds to TM4 of the four membrane-spanning model of Takeshima et al. (13) and TM10 (without N-terminal amino acid residues VVFD) of the 12 membrane-spanning model of Zorzato et al. (14) used by Du et al. (15) to describe an 8 membrane-spanning model. (C) Helical probability of putative C-terminal TM and 22 residues immediately after C-terminal TM was calculated, using the PROF protein sequence analysis program (42,43). In A, the putative RyR1 pore helix, selectivity filter, and linker regions are shown. Bold indicates negatively charged amino acid residues mutagenized to asparagine or glutamine (21, this study). Underlined amino acids indicate residues analogous to the selectivity filter of K+ channels (26).