TABLE 2.
Calculated interaction rates, k2, and the number of close-distance complexes formed for truncated intact cyt f and its large domain interacting with cyt c6
| Intact Cyt f
|
Large domain of Cyt f
|
|||
|---|---|---|---|---|
| No. of complexes /10,000 trajectories |
k2* (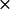 108) M−1 S−1 108) M−1 S−1
|
No. of complexes /10,000 trajectories |
k2* (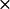 108) M−1 S−1 108) M−1 S−1
|
|
| 1CFM-A | 413±3 | 13.5±0.8 | 37±3 | 1.2±0.2 |
| 1CFM-B | 467±4 | 15.1±0.6 | 41±2 | 1.3±0.2 |
| 1CFM-C | 677±10 | 21.4±0.8 | 110±2 | 3.5±0.3 |
| 1EWH-A | 476±5 | 15.4±0.6 | 69±4 | 2.2±0.2 |
| 1EWH-B | 61±3 | 2.0±0.3 | 1±0.4 | 0.02±0.01 |
| 1EWH-C | 1022±11 | 31.2±0.8 | 163±7 | 5.2±0.5 |
| 1Q90 | 39±3 | 1.3±0.3 | 24±1 | 0.8±0.2 |
| 1Q90-modified† | 366±4 | 12.0±0.6 | – | – |
| 1CFM-C-large‡ domain+K-188 and K-189 | 1220±4 | 35.9±3.1 | – | – |
The close-distance complexes were defined as those with Fe-Fe distances 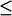 18Å, which are considered electron transfer-active. The second order diffusion-controlled rate constants, k2, were calculated for the formation of these complexes, as described in the Methods section. Five sets of 10,000 trajectories each were carried out to obtain the error values.
18Å, which are considered electron transfer-active. The second order diffusion-controlled rate constants, k2, were calculated for the formation of these complexes, as described in the Methods section. Five sets of 10,000 trajectories each were carried out to obtain the error values.
The structure of 1Q90 cyt f was altered in its small domain, which does not affect the interactions of its large domain with PC.
Only the large domain of 1CFM-C plus residues K-188 and K-189, but without the rest of the small domain, was used. Since a large number of close-distance complexes were formed, we carried only 1000 trajectories.
