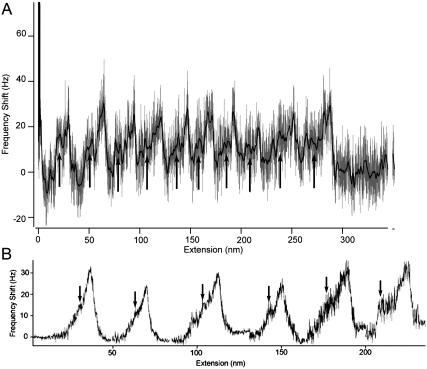
FIGURE 2.
(A) Frequency shift versus extension curve (light gray trace) for the sequential unfolding of titin I27 domains taken using a resonance frequency f of 18.5 kHz and oscillation amplitude A of 6.5 nm. The mean spacing for A values of 4.5 nm, 6.5 nm, and 11.5 nm were 25.2 ± 2.9 (n = 35), 25.3 ± 3.4 (n = 61), and 24.6 ± 6 (n = 13), respectively, indicating the successful unfolding modular domains using this dynamic technique. The curve shows 10 peaks with each peak recording a positive frequency shift of ≈25 Hz. The first two peaks, which are slightly greater in magnitude than the remaining eight, may be due to multiple binding of proteins (i.e., the peaks correspond to an additional protein molecule on the tip). During extension, the increasing stiffness of the unfolded polypeptide region restricts the cantilever movement, causing an increase in the effective spring constant kc of the cantilever. Thus, an increase in kc causes a direct increase in the cantilever resonance frequency, which is observed as a positive frequency shift in the unfolding peaks. A smoothed curve (dark line) clearly shows a discontinuity (arrows) in the positive frequency shift of the main unfolding peak, indicating the unfolding intermediate. (B) Graph showing averaging of all peaks showing intermediates (from A = 4.5 nm and 6.5 nm) and according to their unfolded domain number, which was done by superimposing and aligning each peak at their peak maximum. The distance between peaks is negligible, and the noise in each peak increases with the unfolded domain number due to the fewer numbers of samples averaged. The distance from the beginning of the force transition (arrow) to the peak maximum showed a slight increase with an increase in the unfolded domain number. These distances were calculated to be 5.6 nm (peak 1), 6.6 nm (peak 2), 7.9 nm (peak 3), 8.1 nm (peak 4), 10.9 nm (peak 5), and 14.4 nm (peak 6).