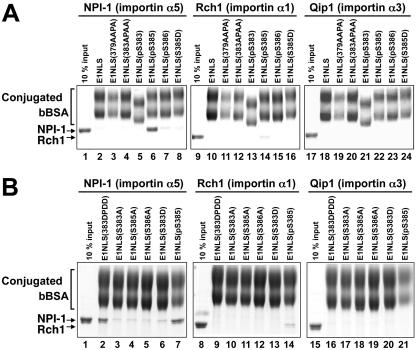
FIG. 7.
Solution-binding assay of interactions of phosphorylated forms and basic amino acids to Ala-substituted forms of EBNA-1 NLS peptides with karyopherin α proteins. (A) Synthetic peptide-cross-linked bBSA proteins bBSA-E1NLS (wild type), bBSA-E1NLS(379AAPA), bBSA-E1NLS(383APAA), bBSA-E1NLS(pS383), bBSA-E1NLS(pS385), bBSA-E1NLS(pS386), and bBSA-E1NLS(S385D), indicated on top of the lanes, were incubated with NPI-1 (importin α5) (lanes 2 to 8), Rch1 (importin α1) (lanes 10 to 16), or Qip1 (importin α3) (lanes 18 to 24) in the presence of avidin-agarose. The bound proteins were eluted and analyzed by SDS-PAGE. Controls are a 10% input of NPI-1 (lane 1), Rch1 (lane 9) and Qip1 (lane 17). (B) Synthetic peptide-cross-linked bBSA proteins bBSA-E1NLS(383DPDD), bBSA-E1NLS(S383A), bBSA-E1NLS(S385A), bBSA-E1NLS(S386A), bBSA-E1NLS(S383D), and bBSA-E1NLS(pS385), indicated at the top of the lanes, were incubated with NPI-1 (importin α5) (lanes 2 to 7), Rch1 (importin α1) (lanes 8 to 14), or Qip1 (importin α3) (lanes 15 to 21) in the presence of avidin-agarose. The bound proteins were eluted and analyzed by SDS-PAGE. Controls are a 10% input of NPI-1 (lane 1), Rch1 (lane 8), and Qip1(lane 15).