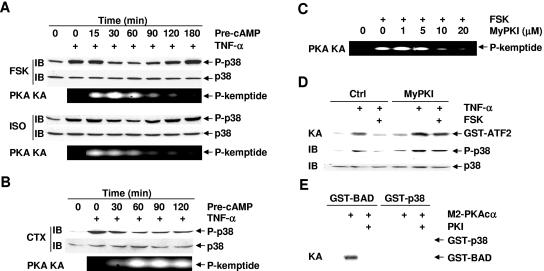
FIG. 2.
cAMP inhibits p38 activation in a PKA-dependent manner. (A and B) Rat-1 cells were pretreated with or without FSK (20 μM), ISO (200 μM), or CTX (100 ng/ml) for various times as indicated, followed by stimulation with or without TNF-α (5 ng/ml, 15 min). Phosphorylation and expression of p38 were analyzed by immunoblotting, and PKA activity was measured by phosphorylation of the fluorogenic kemptide, respectively. (C) Rat-1 cells were pretreated with various doses of the specific PKA inhibitor MyPKI for 30 min, followed by treatment with or without FSK (20 μM, 30 min). PKA activity was determined as in panel A. (D) Rat-1 cells were pretreated with or without MyPKI (10 μM, 30 min) prior to FSK treatment (20 μM, 30 min). After stimulation with TNF-α (5 ng/ml, 15 min), the activity and phosphorylation of p38 were analyzed by immune complex kinase assays and immunoblotting, respectively. (E) Rat-1 cells were transfected with mammalian expression vector encoding M2-PKAcα (4 μg). After 40 h, the activity of M2-PKAcα was measured by immune complex kinase assays with GST-BAD (2 μg) or GST-p38 (2 μg) as substrates. For PKI inhibition, the kinase reaction mixture was incubated with the synthetic peptide inhibitor PKI (1 μg, 30 min) on ice prior to the addition of the ATP mixture. Crtl, control.