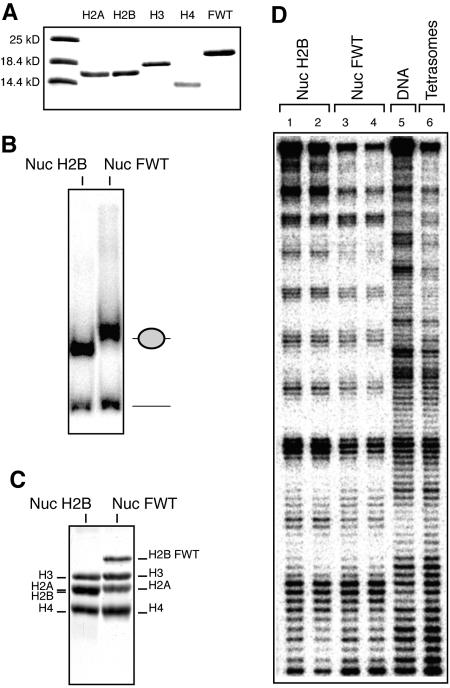
FIG. 2.
The presence of H2BFWT has no effect on the structure of the nucleosome. An equimolar mixture of purified-to-homogeneity recombinant core histones H2A, H3, and H4 and either conventional H2A or histone variant H2BFWT and a 152-bp EcoRI-RsaI 32P-end-labeled DNA fragment containing the X. borealis 5S RNA gene were used to reconstitute nucleosomes. (A) An 18% sodium dodecyl sulfate electrophoresis of conventional core histones and the histone variant H2BFWT. The first lane shows the molecular mass markers. (B) EMSA of the reconstituted conventional (Nuc) and histone variant H2BFWT (Nuc FWT) nucleosomes. The positions of the nucleosomes and the free DNA are shown on the right. (C) Histone composition of the reconstituted nucleosomes. Preparative EMSA was carried out, the conventional and H2BFWT nucleosome bands were cut from the gel, and the nucleoproteins were eluted. Histones were isolated and separated by 18% sodium dodecyl sulfate electrophoresis, and the gel was stained with Coomassie blue. The positions of the histones are indicated. (D) DNase I footprinting of reconstituted conventional H2AB nucleosomes (in duplicate, lanes 1 and 2), variant H2AB nucleosomes (in duplicate, lanes 3 and 4), and tetrasomal particles containing only the (H3-H4)2 tetramer (lane 6). Lane 5, footprinting of naked DNA.