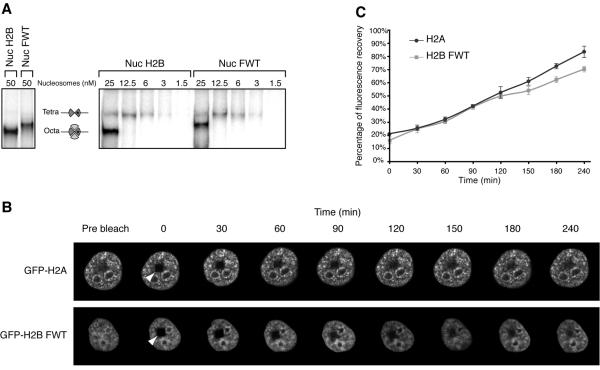
FIG. 4.
In vitro and in vivo stabilities of the variant H2BFWT nucleosome. (A) The H2BFWT variant nucleosome exhibits in vitro the same stability as the conventional H2A nucleosome. Conventional and variant H2BFWT nucleosomes and tetrameric (H3-H4)2 particles were reconstituted by using 32P-labeled histone H3. The samples were diluted to the indicated concentrations with 1× phosphate-buffered saline and run on a native 5.5% polyacrylamide gel. On the left are shown the conventional and H2BFWT nucleosomes at 50 nM. The positions of the intact nucleosomes and tetrameric particles are indicated. Note that upon dilution, the conventional H2A-H2B and the variant H2A-H2BFWT dimers are released with essentially the same efficiency from the reconstituted nucleosomes. (B) FRAP analysis of the mobility of the GFP-H2BFWT variant fusion. Cells stably expressing either GFP-H2A or GFP-H2BFWT were photobleached, and images were recorded at the indicated times postbleaching. The rectangular bleached area is designated by an arrowhead. Images of the cell before bleaching (prebleach) are also shown. (C) Quantification of the data shown in panel B. The means and standard deviations for 45 nuclei from five independent experiments are shown.