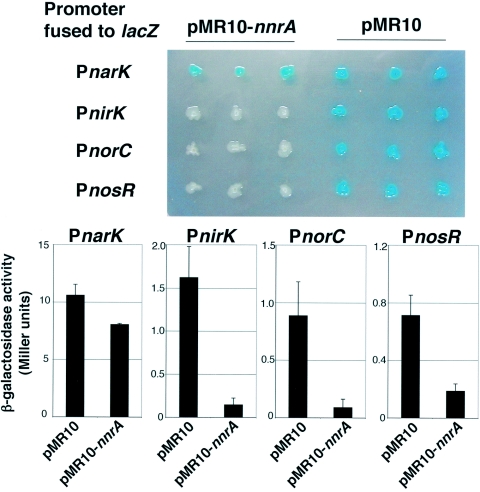
FIG. 2.
Heterologous transcriptional interference test using nnrA of B. melitensis with the narK, nirK, norC, and nosR promoters. E. coli strains contain a pMR10 plasmid bearing nnrA (or a pMR10 without nnrA) and a pBBR-MCS1lacZ derivative, in which a promoter is fused to the lacZ reporter. It is expected that nnrA expression from pMR10-nnrA will interfere with a promoter-lacZ fusion if NnrA is able to modulate the promoter tested, probably by direct binding. In the upper panel, E. coli strains were grown overnight (in aerobic conditions, without the addition of nitrate) on LB medium containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml), chloramphenicol (20 μg/ml), and kanamycin (50 μg/ml) in order to select for strains harboring a pMR10-derived plasmid and pBBRMCS carrying a fusion to the lacZ reporter. In these experiments, nnrA is expressed under the control of an E. coli lac promoter. The strains having the pMR10-nnrA plasmid and the PnirK-lacZ, PnorC-lacZ, and PnosR-lacZ fusions are less stained than the controls (pMR10-containing strains and the strain containing pMR10-nnrA and the PnarK-lacZ fusion). Three independent clones are shown. These interferences of nnrA with nirK, norC, and nosR promoters that were observed with X-Gal staining are confirmed by a standard β-galactosidase (β-gal) assay (lower panel) made with clones grown overnight in liquid LB medium supplemented with chloramphenicol (20 μg/ml) and kanamycin (50 μg/ml) (8). The indicated values are the averages of measurements of three independent clones, and each error bar corresponds to one standard deviation.