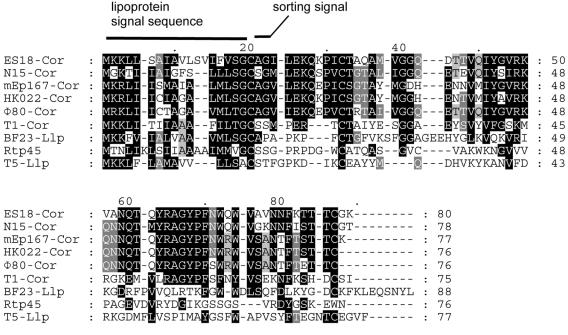
FIG. 3.
Alignment of Cor proteins with other predicted small phage lipoproteins. This multiple-sequence alignment of full-length protein sequences was generated with ClustalW. The fully conserved cysteine residue at position 20 of the alignment constitutes the N-terminal amino acid of the mature lipoproteins. It is the site for cleavage of the signal sequence and lipid modification. Llp of phage T5 is the only one of these proteins for which lipid modification and its functional importance have been experimentally proven (28, 68, 77).The Cor protein of phage T1 is the most distant member of the Cor family, which consists of a group of six homologous sequences. Llp of T5 and BF23 and Rtp45 show little sequence conservation with respect to the Cor proteins beyond the signal and sorting sequences. The correct amino acid sequence for Cor of Φ80 has been taken from Vostrov et al. (88). Accession numbers of the other Cor and Llp proteins: ES18, AAW70503; N15, NP_046919; mEp167, AAT11800; HK022, NP_037685; T1, AAP49969; T5, Q38162; BF23, AAZ03643.