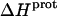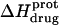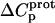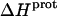TABLE 3.
Temperature dependent enthalpy and corresponding heat capacity changes for protonation of the five paromomycin amino groups
| Amino group |
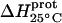 * (kcal/mol) * (kcal/mol) |
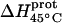 * (kcal/mol) * (kcal/mol) |
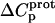 † (cal/mol K) † (cal/mol K) |
|---|---|---|---|
| 1 | −15.8 ± 3.4 | −7.9 ± 1.4 | +392 ± 244 |
| 3 | −9.0 ± 0.1 | −8.2 ± 0.1 | +38 ± 4 |
| 2′ | −11.5 ± 5.0 | −12.7 ± 3.6 | −58 ± 430 |
| 2‴ | −5.4 ± 1.7 | −7.3 ± 2.2 | −93 ± 199 |
| 6‴ | −9.4 ± 0.1 | −8.4 ± 0.1 | +48 ± 12 |