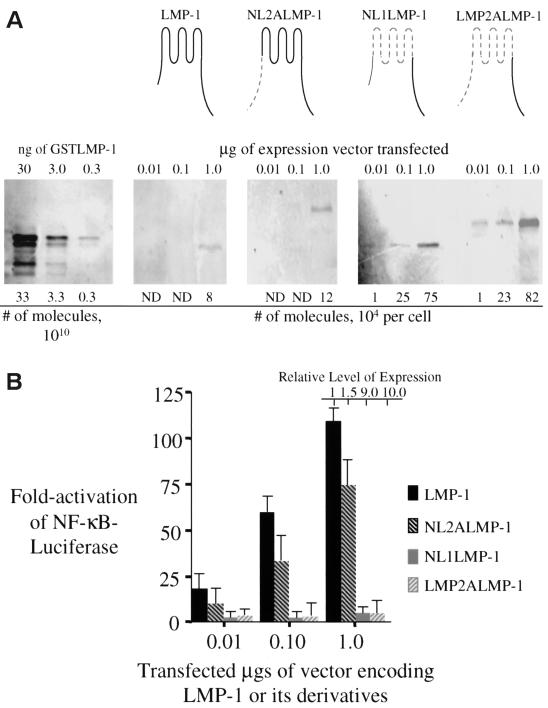
FIG. 1.
LMP-1 and its derivatives with substitutions in its amino terminus and membrane-spanning domains were tested for their ability to activate NF-κB-mediated transcription. (A) Quantitative Western blots were performed to measure the levels of expression of LMP-1 (aa 1 to 386 [depicted in black]), NL2ALMP-1 (LMP-1's N terminus substituted with LMP-2A's N terminus [depicted by gray dashed lines]), NL1ALMP-1 (LMP-1's six transmembrane domain, including its intracellular and extracellular loops substituted with LMP-2A's first six transmembrane domains, including the intracellular and extracellular loops [depicted by gray dashed lines]), and LMP-2ALMP-1 (LMP-1's N terminus and transmembrane domains substituted with LMP-2A's N terminus and transmembrane domain, including its intracellular and extracellular loops [depicted by gray dashed lines]) after the vectors encoding them and one for GFP were introduced into 293 cells. A total of 105 GFP-positive cells transfected with the indicated amount of expression vector for each expression plasmid was lysed and separated electrophoretically by SDS-PAGE. The samples were transferred to a nitrocellulose membrane and then probed with a rabbit anti-LMP-1 antibody, a secondary biotinylated goat anti-rabbit antibody, and 35S-labeled streptavidin. The samples were visualized and quantified by phosphorimage analysis. The number of molecules of LMP-1 and its derivatives were calculated from known amounts of GSTLMP-1 assayed on the same blot. (B) The stimulation of NF-κB activity in 293 cells transfected with the amount of expression vectors indicated for LMP-1, NL1LMP-1, NL2ALMP-1, and LMP-2ALMP-1 was measured with an NF-κB responsive luciferase reporter. All transfections were normalized to renilla luciferase levels or to the number of GFP-positive cells. The expression of each protein relative to that of wtLMP-1 is shown and was determined from Fig. 1A. The fold activation of firefly luciferase over cells transfected with pSG5 alone is shown. The relative light units (RLUs) in these experiments varied from ∼5.0 × 103 to ∼2.0 × 104 in cells transfected with empty vector and up to ∼3.0 × 106 in the presence of expression vectors for LMP-1 or its derivatives. Dividing the fold activation by the relative level of expression for LMP-1 and each of its derivatives gives the fold activation on a per molecule basis for each of the proteins tested. The data represent the average ± the standard deviation for three separate experiments with two measurements each.