Abstract
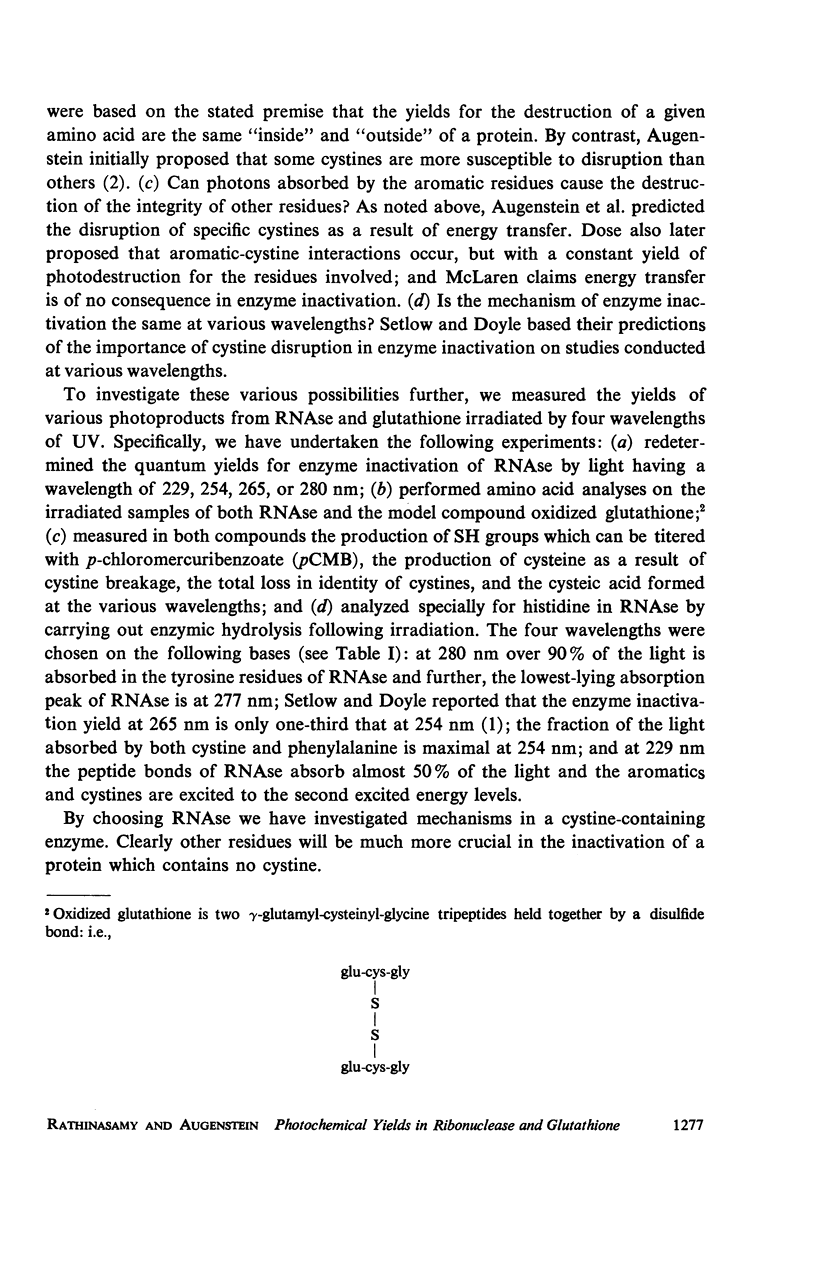
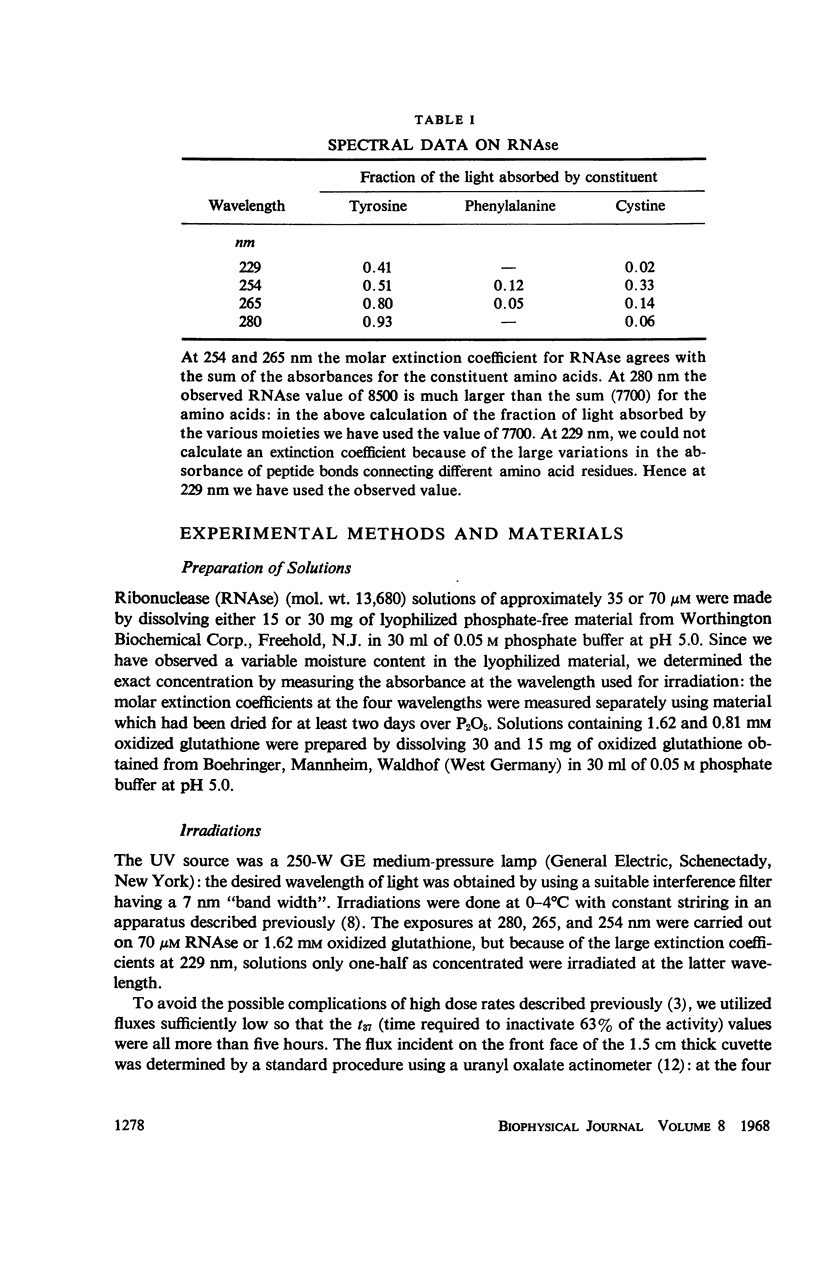
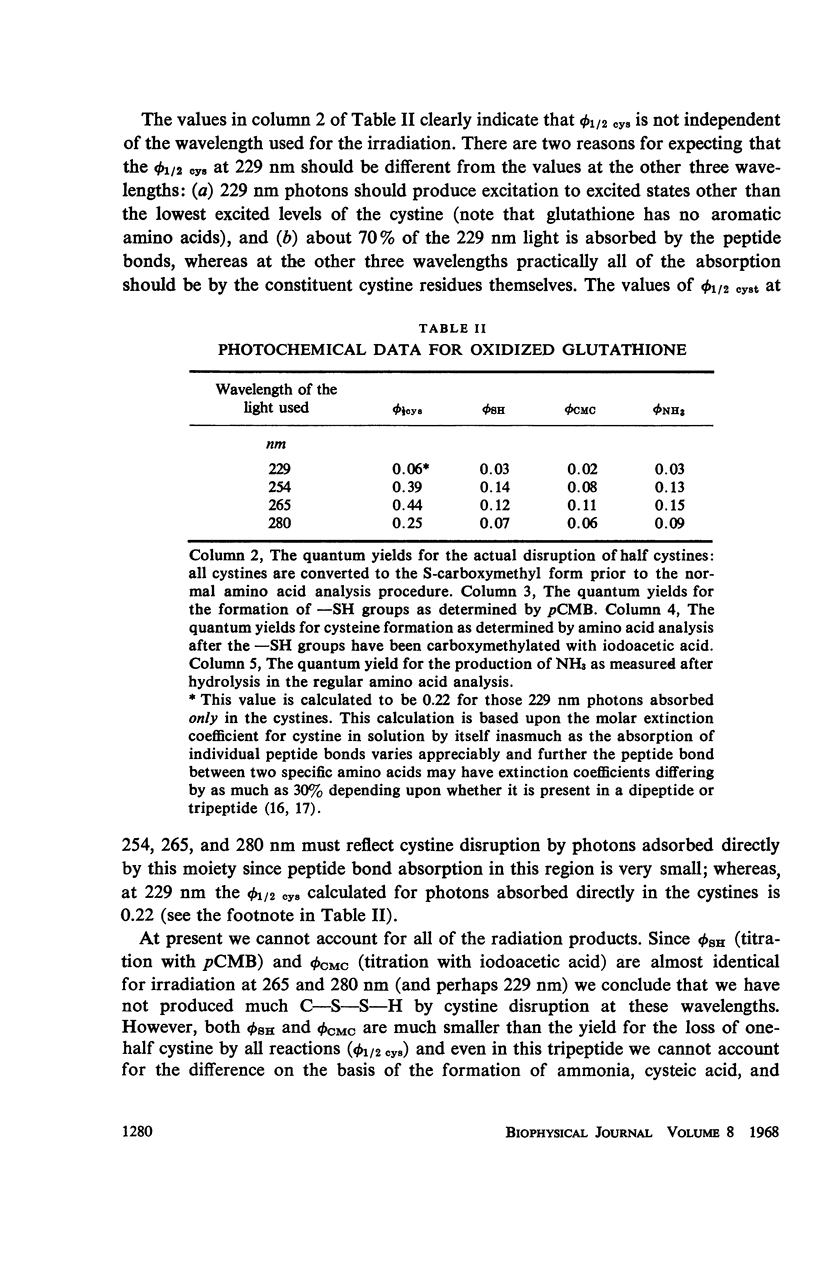
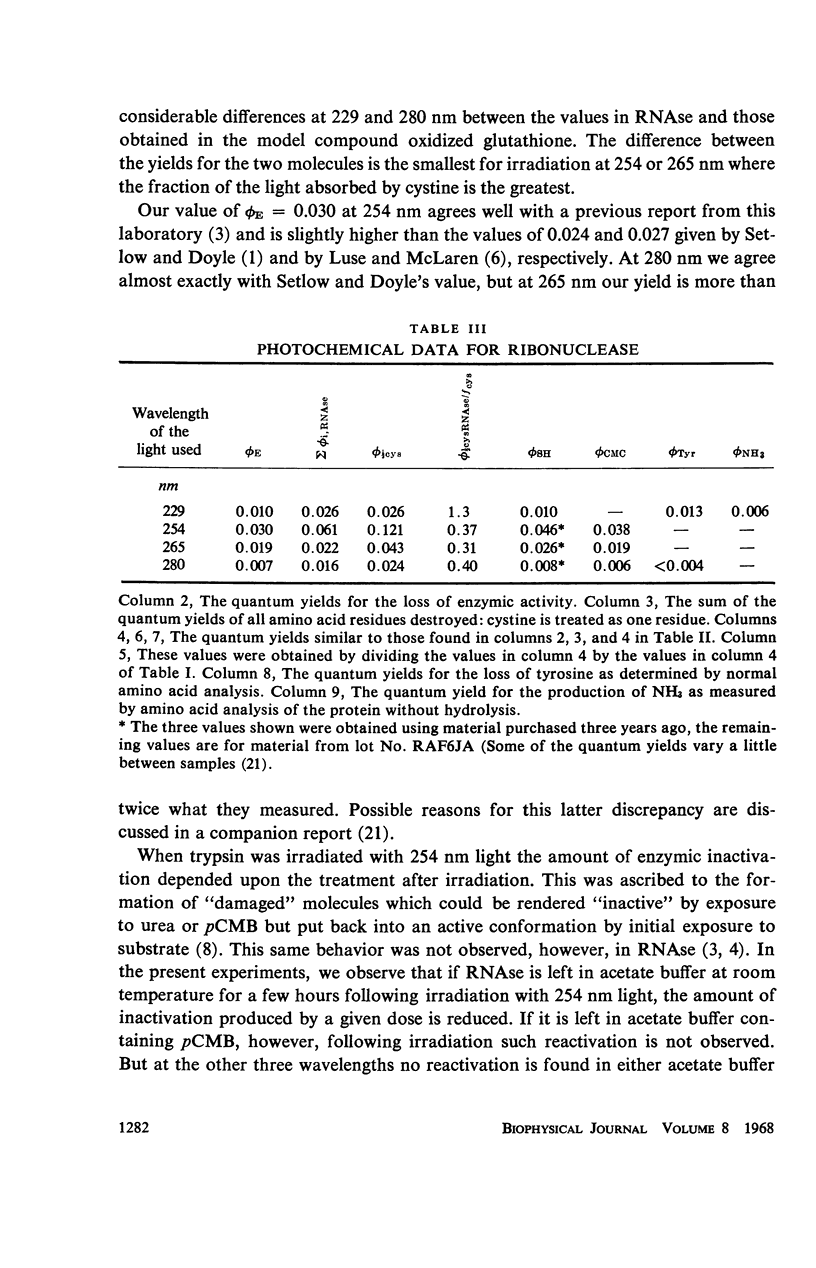
The quantum yields for the disruption of various amino acids in glutathione and ribonuclease by 229, 254, 265, and 280 nm UV photons have been determined. The results of the measurements on the destruction of tyrosine and histidine and the loss of enzymic function in RNAse and the disruption of cystine in both compounds lead to the following conclusions: (a) The photodestruction of some and perhaps many constituent amino acid residues does not cause RNAse inactivation. (b) Contrary to the basic premise of proposals made by other authors, the photochemical yields of constituent residues in a protein are not the same as that for the same amino acids in solution alone—the difference is a function of the exciting wavelength. Further, the extent of histidine destruction varies by a large factor among three proteins. (c) Consistent with previous predictions, the present results show that photons absorbed in the aromatic residues of RNAse cause the disruption of cystines elsewhere in the enzyme. (d) Although cystine disruption appears to be the most prevalent mode of RNAse inactivation by photons of the four wavelengths studied, some of the minor mechanisms leading to loss of enzymic function may vary with the UV energy.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGENSTINE L. G., GHIRON C. A. The inactivation of trypsin by ultraviolet light. I. The correlation of inactivation with the disruption of constituent cystine. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1530–1547. doi: 10.1073/pnas.47.10.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRINI U. CHANGES IN AMINO ACID CONTENT AND INACTIVATION OF LYSOZYME FOLLOWING ULTRAVIOLET IRRADIATION. Arch Biochem Biophys. 1964 Jul;107:126–131. doi: 10.1016/0003-9861(64)90278-4. [DOI] [PubMed] [Google Scholar]
- FERRINI U., ZITO R. MODIFICATION OF HISTIDINE BY ULTRAVIOLET IRRADIATION OF LYSOZYME. J Biol Chem. 1963 Nov;238:3824–3825. [PubMed] [Google Scholar]
- GUNDLACH H. G., STEIN W. H., MOORE S. The nature of the amino acid residues involved in the inactivation of ribonuclease by iodoacetate. J Biol Chem. 1959 Jul;234(7):1754–1760. [PubMed] [Google Scholar]
- Grist K. L., Taylor T., Augenstein L. The inactivation of enzymes by ultraviolet light. V. The disruption of specific cystines in ribonuclease. Radiat Res. 1965 Oct;26(2):198–210. [PubMed] [Google Scholar]
- KALNITSKY G., HUMMEL J. P., RESNICK H., CARTER J. R., BARNETT L. B., DIERKS C. The relation of structure to enzymatic activity in ribonuclease. Ann N Y Acad Sci. 1959 Sep 4;81:542–569. doi: 10.1111/j.1749-6632.1959.tb49336.x. [DOI] [PubMed] [Google Scholar]
- Piras R., Vallee B. L. Effect of ultraviolet irradiation on composition and function of carboxypeptidase A. Biochemistry. 1966 Mar;5(3):849–854. doi: 10.1021/bi00867a006. [DOI] [PubMed] [Google Scholar]
- Risi S., Dose K., Rathinasamy T. K., Augenstein L. The effect of environment on cystine disruption by ultraviolet light. Photochem Photobiol. 1967 Jun;6(6):423–436. doi: 10.1111/j.1751-1097.1967.tb08889.x. [DOI] [PubMed] [Google Scholar]
- Risi S., Dose K., Rathinasamy T. K., Augenstein L. The effect of environment on cystine disruption by ultraviolet light. Photochem Photobiol. 1967 Jun;6(6):423–436. doi: 10.1111/j.1751-1097.1967.tb08889.x. [DOI] [PubMed] [Google Scholar]
- SETLOW R., DOYLE B. The action of monochromatic ultraviolet light on proteins. Biochim Biophys Acta. 1957 Apr;24(1):27–41. doi: 10.1016/0006-3002(57)90142-7. [DOI] [PubMed] [Google Scholar]
- Yeargers E., Bishai F. R., Augenstein L. On the nature of tyrosine phosphorescence from proteins. Biochem Biophys Res Commun. 1966 May 25;23(4):570–575. doi: 10.1016/0006-291x(66)90768-6. [DOI] [PubMed] [Google Scholar]


