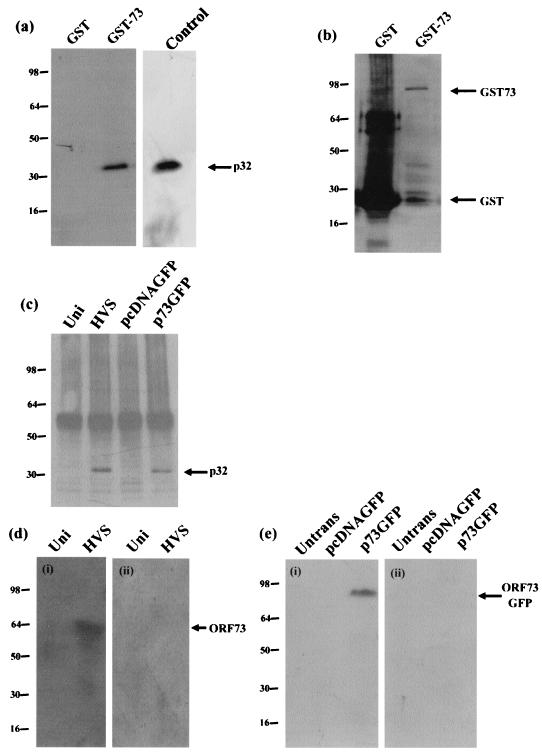
FIG. 1.
The ORF 73 protein interacts with the cellular protein p32. (a) Control GST alone and GST-ORF 73 were expressed in E. coli and purified from crude lysate by incubation with glutathione-Sepharose 4B affinity beads. Protein extracts of untransfected cells were incubated with GST or GST-ORF 73 proteins. Bound proteins were resolved by SDS-PAGE (12% polyacrylamide), and p32 was detected by Western blotting using p32 antiserum. A control lane of whole-cell lysate is shown to estimate the level of interaction between p32 and GST-73. (b) To confirm the expression of GST and GST-ORF 73 proteins, Western blot analysis was performed using a mouse monoclonal GST antibody. (c) Mock-infected (Uni), HVS-infected, pcDNAGFP-transfected, or p73GFP-transfected cell extracts were incubated with ORF 73 polyclonal antiserum bound to protein A-Sepharose beads. The beads were then pelleted and washed, and precipitated polypeptides were resolved on an SDS-12% polyacrylamide gel and analyzed by immunoblot analysis with p32 antiserum. (d) Mock-infected OMK (Uni) and HVS-infected cell extracts were incubated with rp32-Sepharose (i) or rGO-Sepharose (ii). After being washed and eluted, the cells were separated by SDS-PAGE and analyzed by Western blotting with ORF 73 polyclonal antisera. (e) Untransfected Cos-7 and pcDNAGFP-transfected or p73GFP-transfected cell extracts were incubated with rp32-Sepharose (i) or rGO-Sepharose (ii). After being washed and eluted, the cells were separated by SDS-PAGE and analyzed by Western blotting with GFP monoclonal antisera.