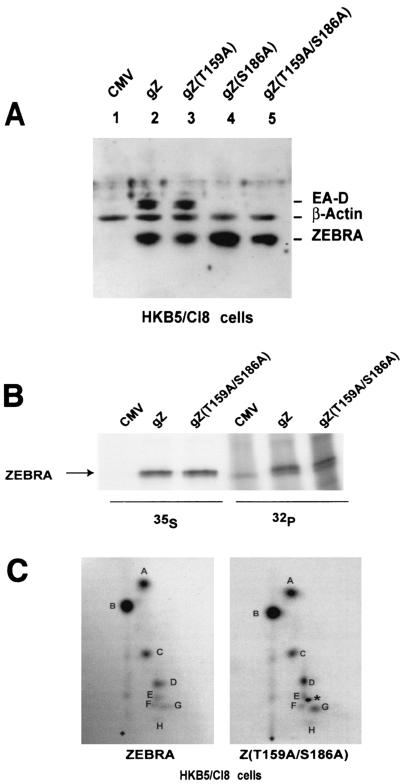
FIG. 5.
Alanine mutations at T159 and S186 do not affect the phosphorylation state of ZEBRA in vivo in HKB5/Cl8 cells. (A) Capacities of wild-type and mutant ZEBRA proteins to activate the EBV BMRF1 gene (EA-D) in HKB5/Cl8 cells. Cells (2 × 106) were transfected with 4 μg of plasmids expressing ZEBRA, Z(T159A), Z(S186A), or Z(T159A/S186A). The cells were harvested 24 h after transfection, and the cell lysates were separated by SDS-10% PAGE and immunoblotted using specific antibodies against the indicated proteins. gZ, genomic ZEBRA. (B) Immunoprecipitation of 35S- and 32P-labeled ZEBRA. HKB5/Cl-8 cells were transfected with empty vector (CMV) or vectors expressing wild-type ZEBRA (gZ) or Z(T159A/S186A). Twelve hours after transfection, the cells were incubated in medium containing both [35S]methionine and [35S]cysteine or [32P]orthophosphate for 7 h. The ZEBRA proteins were immunoprecipitated from the cell extracts and separated by SDS-8% PAGE. The gel was dried and autoradiographed. (C) Two-dimensional tryptic phosphopeptide maps of wild-type ZEBRA and Z(T159A/S186A). The 32P-labeled wild-type and mutant ZEBRA proteins were excised and extracted from the gel shown in panel B and digested with trypsin. The peptides generated by trypsin were separated in two dimensions on thin-layer cellulose. After autoradiography, eight phosphopeptides were detected, designated A to H. No difference was observed between the phosphopeptide map of wild-type ZEBRA and that of the double mutant. One extra dark spot (∗) among the Z(T159A/S186A) phosphopeptides between spots E and F is an autoradiography artifact.