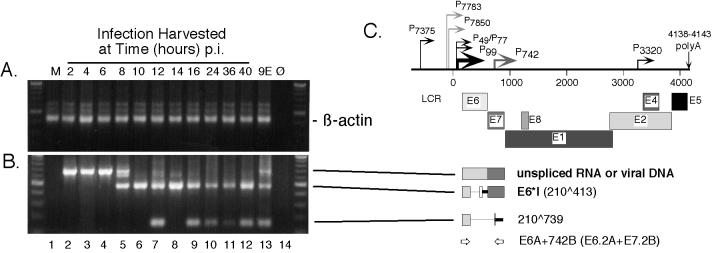
FIG. 3.
RT-nested-PCR analysis of β-actin and spliced HPV31b E6 transcripts expressed following infection of HaCaT cells. Subconfluent HaCaT monolayers were infected with an HPV31b stock at 20 vDNA-containing particles per cell. The cells were harvested for total RNAs at 2-h intervals beginning at 2 h p.i. and continuing to 16 p.i. Infected cells were also harvested at 8-h intervals up to 40 h p.i. For each sample an RT reaction was performed using 12.0 μg of DNase I-treated total RNA. Samples included mock-infected HaCaT cells (M); HaCaT cells harvested at 2, 4, 6, 8, 10, 12, 14, 16, 24, 36, 48 h p.i; and CIN-612 9E monolayers (9E). No RNA input (Ø) served as a negative amplification control. RT assays were divided into seven different first round PCR amplifications of 45 cycles using primer sets A, B, C, D, E, and F from Fig. 1 and also targeting spliced cellular β-actin. Following the first round of PCR, 4% of each of these assays was used in nested PCR of 30 cycles. (A) Primers β-actin OA and β-actin OB were used in the first round, and primers β-actin IA and β-actin IB were used in nested PCR to detect a 429-bp amplimer derived from spliced β-actin RNA. Input RNA in the first round of PCR corresponded to 380 ng. (B) Primers E6A and 742B were used in the first round, primers E6.2A and E7.2B were used in nested PCR. For each reaction, the input RNA in the first round of PCR corresponded to 1.9 μg. Molecular size standards (100-bp ladder; New England Biolabs) are shown at the edges of each panel. (C) Early region of the HPV31b genome as described in the legend to Fig. 1. Below the genome diagram, the structures of the observed amplicons derived from viral RNAs or DNAs are shown with lines pointing to the respective amplicons in panels A and B. Boldface letters denote previously characterized ORFs, with their splice junctions indicated in parentheses. The newly identified RNA structure containing the 210∧739 splice junction is indicated. Both spliced structures were verified by sequencing from at least one of the samples shown. Placement and orientation of HPV31b PCR primers are given with primer pairs used for the first round of PCR indicated first. Primer pairs used in nested PCR are given in parentheses.