Abstract
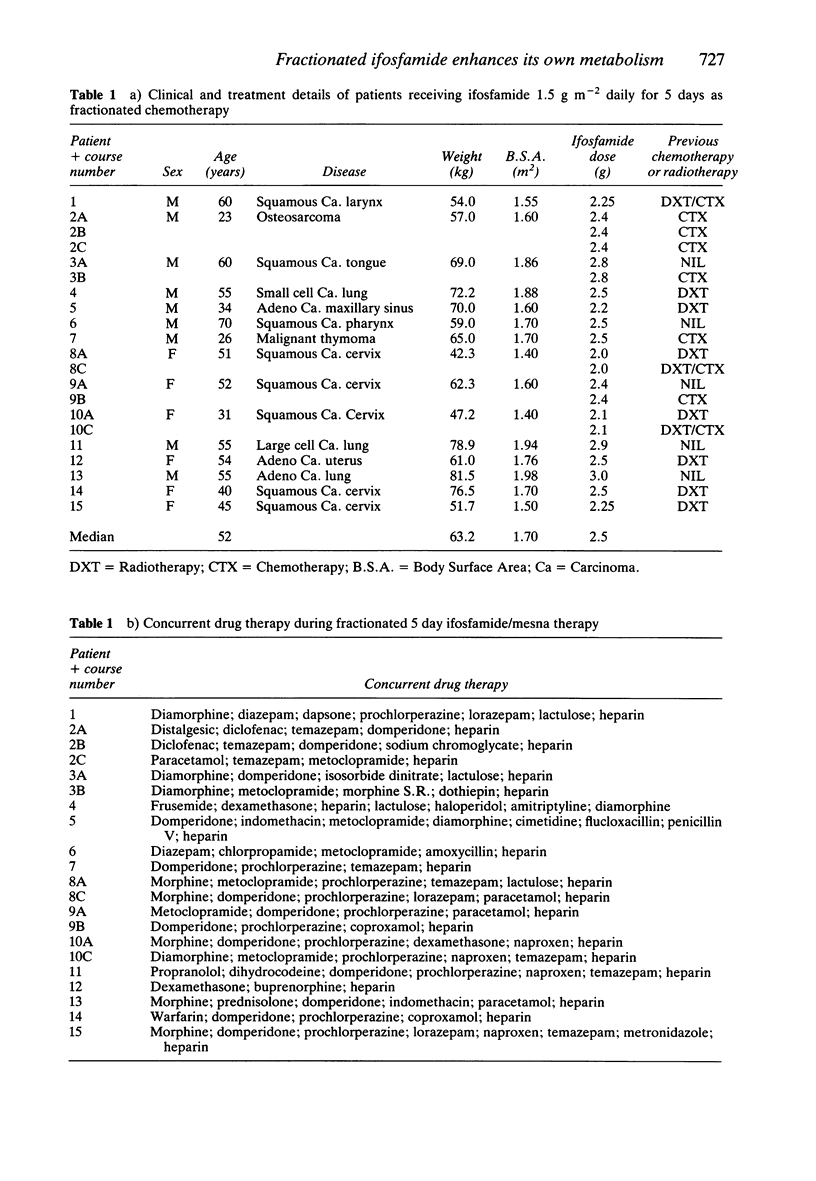
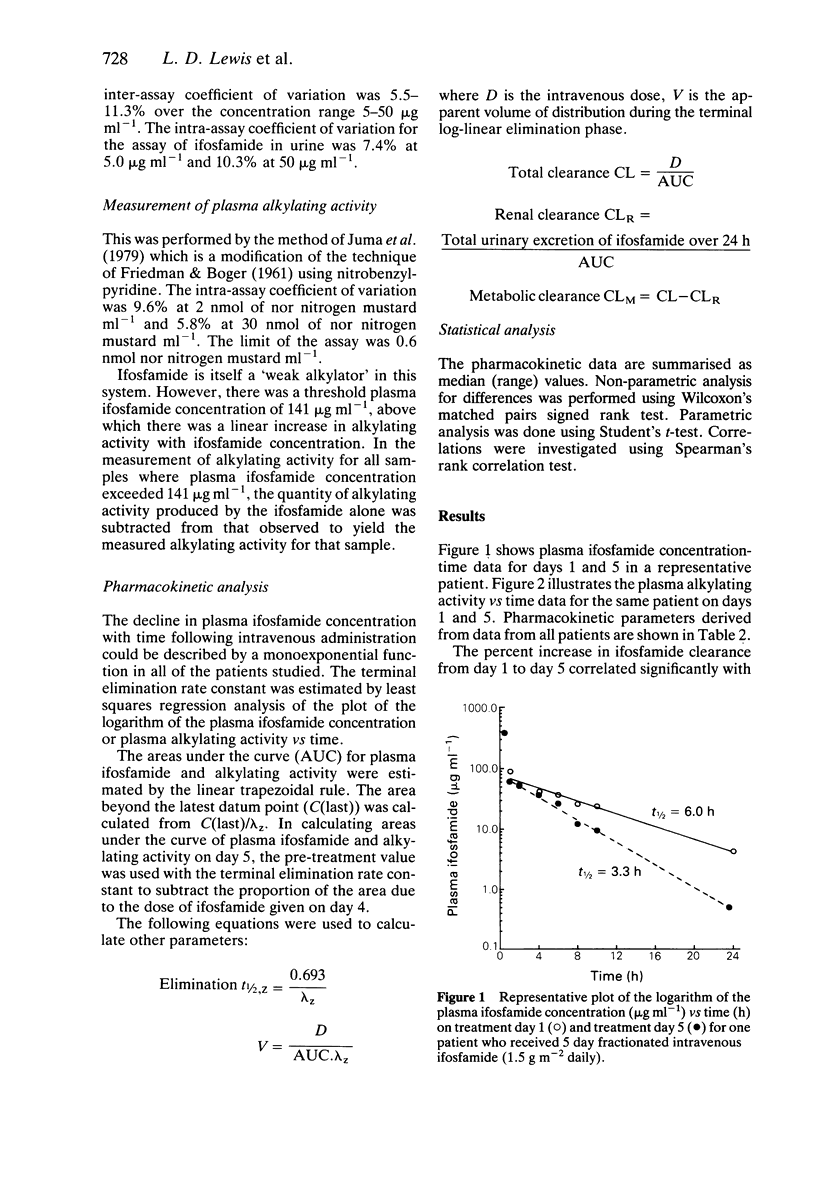
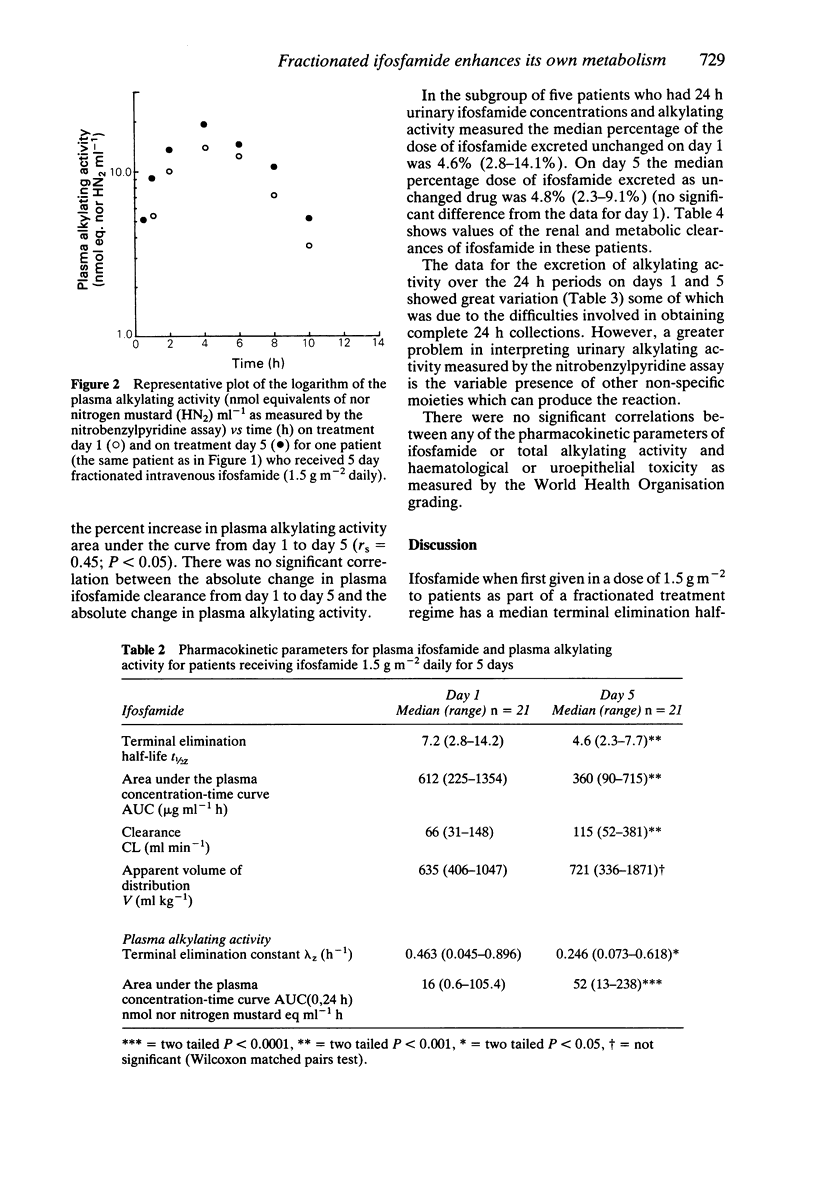
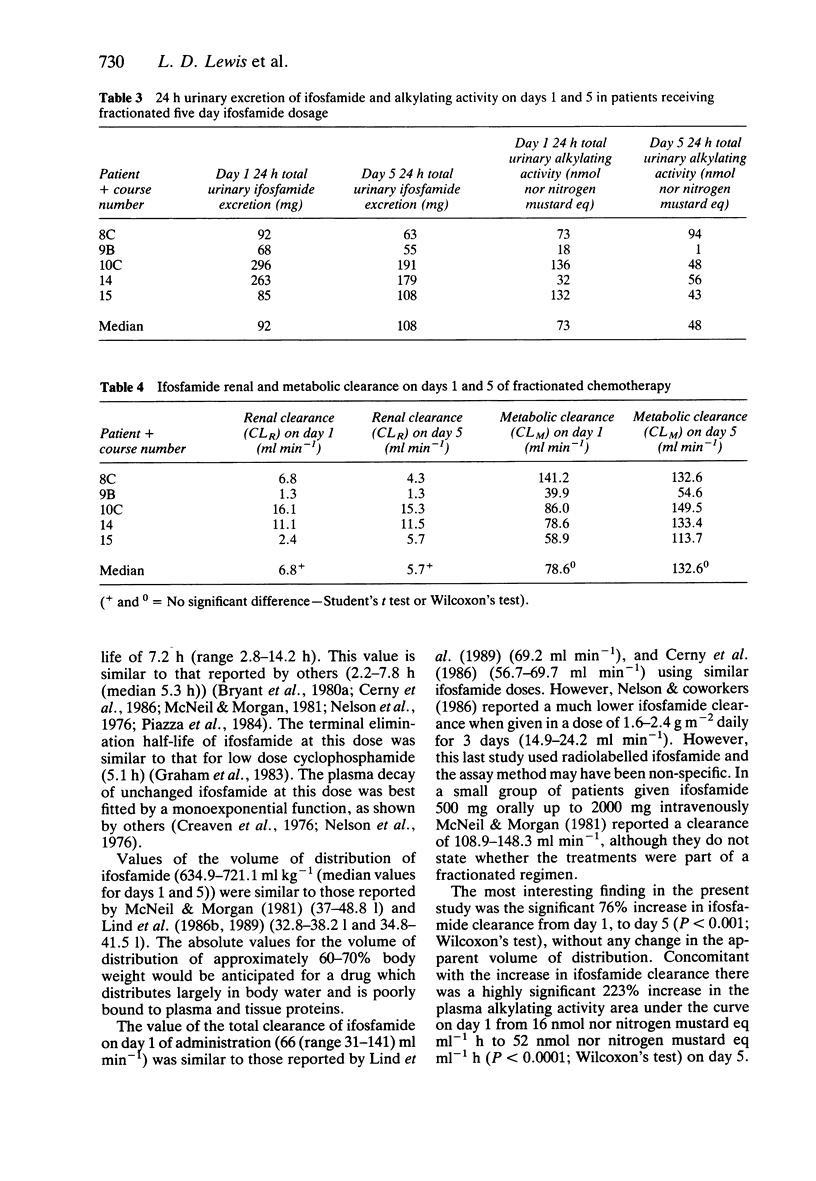
1. Fifteen patients received 1.5 g m-2 of ifosfamide intravenously over 0.5 h every day for 5 days. Twenty-one courses of treatment were studied. Plasma was assayed for ifosfamide by gas liquid chromatography and plasma alkylating activity was measured using the nitrobenzylpyridine (NBP) reaction. 2. A pharmacokinetic analysis revealed a significant decrease in the median (range) elimination half-life of ifosfamide from 7.2 (2.8-14.2) h on day 1 to 4.6 (2.3-7.7) h on day 5 (P less than 0.001, Wilcoxon's test) with a concomitant significant increase in the median (range) clearance from 66 (31-148) ml min-1 on day 1 to 115 (52-381) ml min-1 on day 5 (P less than 0.001). There was no significant change in the volume of distribution on day 5 compared with day 1. 3. There was a highly significant 223% increase in the median (range) plasma nitrobenzylpyridine alkylating activity area under the curve on day 1 from 16 (0.6-105) nmol nor nitrogen mustard equivalents ml-1 h to 52 (13-238) nmol nor nitrogen mustard equivalents ml-1 h on day 5. 4. During five courses of treatment (in five patients in the group) 24 h urine samples were collected on days 1 and 5. The median (range) renal clearance of ifosfamide on day 1 was 6.8 (1.3-16.2) ml min-1 compared with 5.7 (1.3-15.3) ml min-1 on day 5. This difference was not significant. The median (range) metabolic clearance of ifosfamide in these five patients on day 1 was 78.6 (39.9-141.2) ml min-1 and 132.6 (54.6-149.5) ml min-1 on day 5.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon R. A., Meienhofer J., Atherton E. Isophosphamide as a new acrolein-producing antineoplastic isomer of cyclophosphamide. Cancer Res. 1972 Nov;32(11):2519–2523. [PubMed] [Google Scholar]
- Allen L. M., Creaven P. J. Activation of the antineoplastic drug isophosphamide by rat liver microsomes. J Pharm Pharmacol. 1972 Jul;24(7):585–586. doi: 10.1111/j.2042-7158.1972.tb09065.x. [DOI] [PubMed] [Google Scholar]
- Brade W. P., Herdrich K., Varini M. Ifosfamide--pharmacology, safety and therapeutic potential. Cancer Treat Rev. 1985 Mar;12(1):1–47. doi: 10.1016/0305-7372(85)90011-8. [DOI] [PubMed] [Google Scholar]
- Bryant B. M., Jarman M., Ford H. T., Smith I. E. Prevention of isophosphamide-induced urothelial toxicity with 2-mercaptoethane sulphonate sodium (mesnum) in patients with advanced carcinoma. Lancet. 1980 Sep 27;2(8196):657–659. doi: 10.1016/s0140-6736(80)92703-8. [DOI] [PubMed] [Google Scholar]
- Cerny T., Margison J. M., Thatcher N., Wilkinson P. M. Bioavailability of ifosfamide in patients with bronchial carcinoma. Cancer Chemother Pharmacol. 1986;18(3):261–264. doi: 10.1007/BF00273399. [DOI] [PubMed] [Google Scholar]
- Creaven P. J., Allen L. M., Cohen M. H., Nelson R. L. Studies on the clinical pharmacology and toxicology of isophosphamide (NSC-109724). Cancer Treat Rep. 1976 Apr;60(4):445–449. [PubMed] [Google Scholar]
- D'Incalci M., Bolis G., Facchinetti T., Mangioni C., Morasca L., Morazzoni P., Salmona M. Decreased half life of cyclophosphamide in patients under continual treatment. Eur J Cancer. 1979 Jan;15(1):7–10. doi: 10.1016/0014-2964(79)90198-1. [DOI] [PubMed] [Google Scholar]
- Drings P., Allner R., Brock N., Burkert H., Fischer M., Fölsch E., Gerhartz H., Götzky P., Hoppe I., Kanzler G. Erfahrungen mit neuartigen N-Lost-Phosphamidestern. Dtsch Med Wochenschr. 1970 Mar 6;95(10):491–497. doi: 10.1055/s-0028-1108490. [DOI] [PubMed] [Google Scholar]
- Gibson G. G., Tamburini P. P. Cytochrome P-450 spin state: inorganic biochemistry of haem iron ligation and functional significance. Xenobiotica. 1984 Jan-Feb;14(1-2):27–47. doi: 10.3109/00498258409151397. [DOI] [PubMed] [Google Scholar]
- Graham M. I., Shaw I. C., Souhami R. L., Sidau B., Harper P. G., McLean A. E. Decreased plasma half-life of cyclophosphamide during repeated high-dose administration. Cancer Chemother Pharmacol. 1983;10(3):192–193. doi: 10.1007/BF00255760. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L., Gessner T., Culliton P. Studies of the effects of cyclophosphamide, vincristine, and prednisone on some hepatic oxidations and conjugations. Cancer Treat Rep. 1976 Sep;60(9):1285–1294. [PubMed] [Google Scholar]
- Hill D. L., Laster W. R., Jr, Kirk M. C., el-Dareer S., Struck R. F. Metabolism of iphosphamide (2-(2-chloroethylamino)-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide) and production of a toxic iphosphamide metabolite. Cancer Res. 1973 May;33(5):1016–1022. [PubMed] [Google Scholar]
- Juma F. D., Rogers H. J., Trounce J. R., Bradbrook I. D. Pharmacokinetics of intravenous cyclophosphamide in man, estimated by gas-liquid chromatography. Cancer Chemother Pharmacol. 1978;1(4):229–231. doi: 10.1007/BF00257155. [DOI] [PubMed] [Google Scholar]
- Juma F. D., Rogers H. J., Trounce J. R. Pharmacokinetics of cyclophosphamide and alkylating activity in man after intravenous and oral administration. Br J Clin Pharmacol. 1979 Sep;8(3):209–217. doi: 10.1111/j.1365-2125.1979.tb01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind M. J., Margison J. M., Cerny T., Thatcher N., Wilkinson P. M. Comparative pharmacokinetics and alkylating activity of fractionated intravenous and oral ifosfamide in patients with bronchogenic carcinoma. Cancer Res. 1989 Feb 1;49(3):753–757. [PubMed] [Google Scholar]
- Lipton A., Hepner G. W., White D. S., Harvey H. A. Decreased hepatic drug demethylation in patients receiving chemo-immunotherapy. Cancer. 1978 May;41(5):1680–1684. doi: 10.1002/1097-0142(197805)41:5<1680::aid-cncr2820410506>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- McNiel N. O., Morgan L. R., Jr The bioavailability of oral and intravenous ifosfamide in the treatment of bronchogenic carcinoma. Int J Clin Pharmacol Ther Toxicol. 1981 Nov;19(11):490–493. [PubMed] [Google Scholar]
- Morgan L. R., Harrison E. F., Hawke J. E., Hunter H. L., Costanzi J. J., Plotkin D., Tucker W. G., Worrall P. M. Toxicity of single- vs. fractionated-dose ifosfamide in non-small cell lung cancer: a multi-center study. Semin Oncol. 1982 Dec;9(4 Suppl 1):66–70. [PubMed] [Google Scholar]
- Nelson R. L., Allen L. M., Creaven P. J. Pharmacokinetics of divided-dose ifosfamide. Clin Pharmacol Ther. 1976 Mar;19(3):365–370. doi: 10.1002/cpt1976193365. [DOI] [PubMed] [Google Scholar]
- Piazza E., Cattaneo M. T., Varini M. Pharmacokinetic studies in lung cancer patients. Cancer. 1984 Sep 15;54(6 Suppl):1187–1192. doi: 10.1002/1097-0142(19840915)54:1+<1187::aid-cncr2820541316>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Rodriguez V., Bodey G. P., Freireich E. J., McCredie K. B., McKelvey E. M., Tashima C. K. Reduction of ifosfamide toxicity using dose fractionation. Cancer Res. 1976 Aug;36(8):2945–2948. [PubMed] [Google Scholar]
- Teunissen M. W., Willemse P. H., Sleijfer D. T., Sluiter W. J., Breimer D. D. Antipyrine metabolism in patients with disseminated testicular cancer and the influence of cytostatic treatment. Cancer Chemother Pharmacol. 1984;13(3):181–185. doi: 10.1007/BF00269025. [DOI] [PubMed] [Google Scholar]


