Abstract
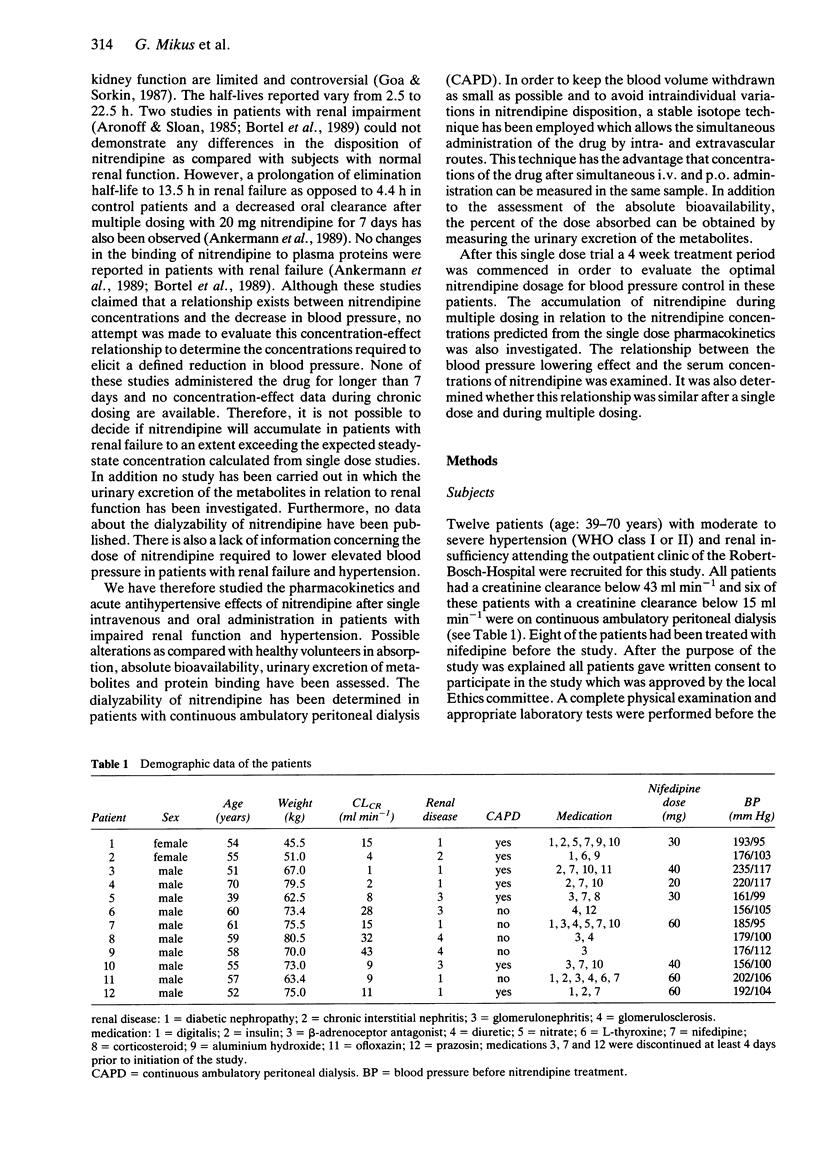
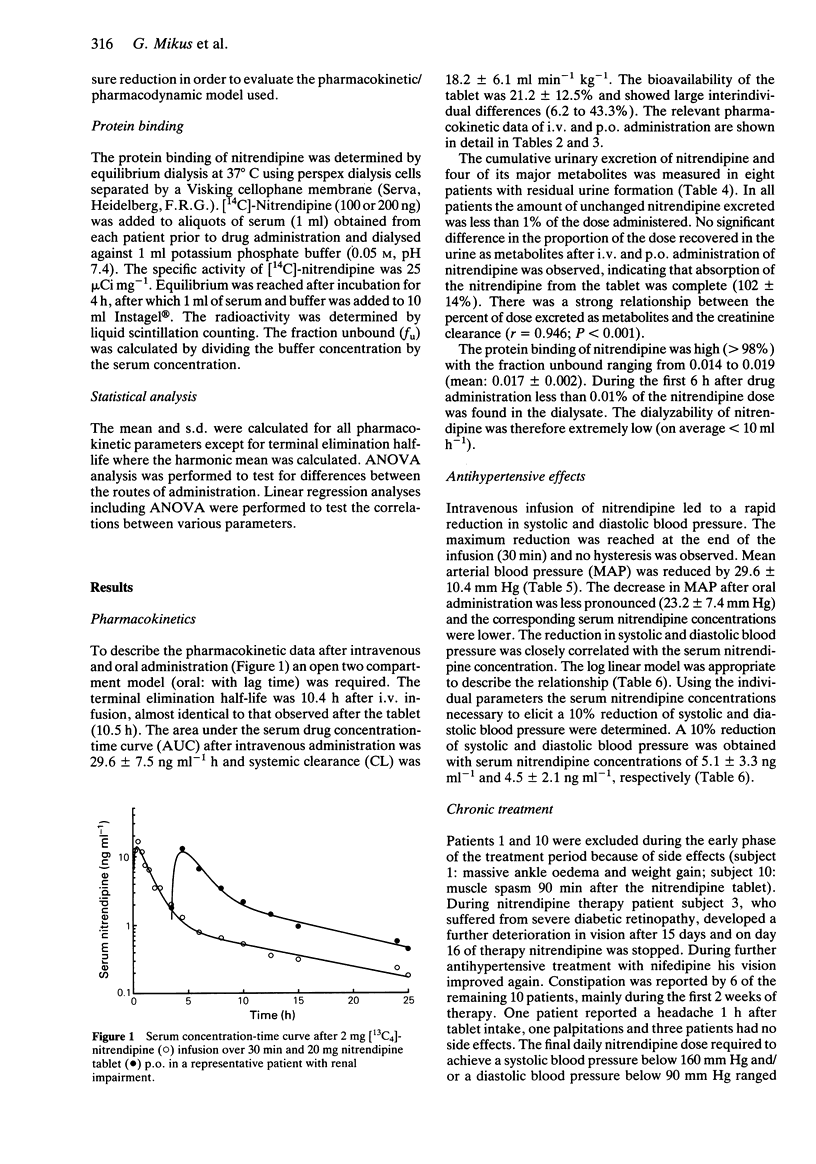
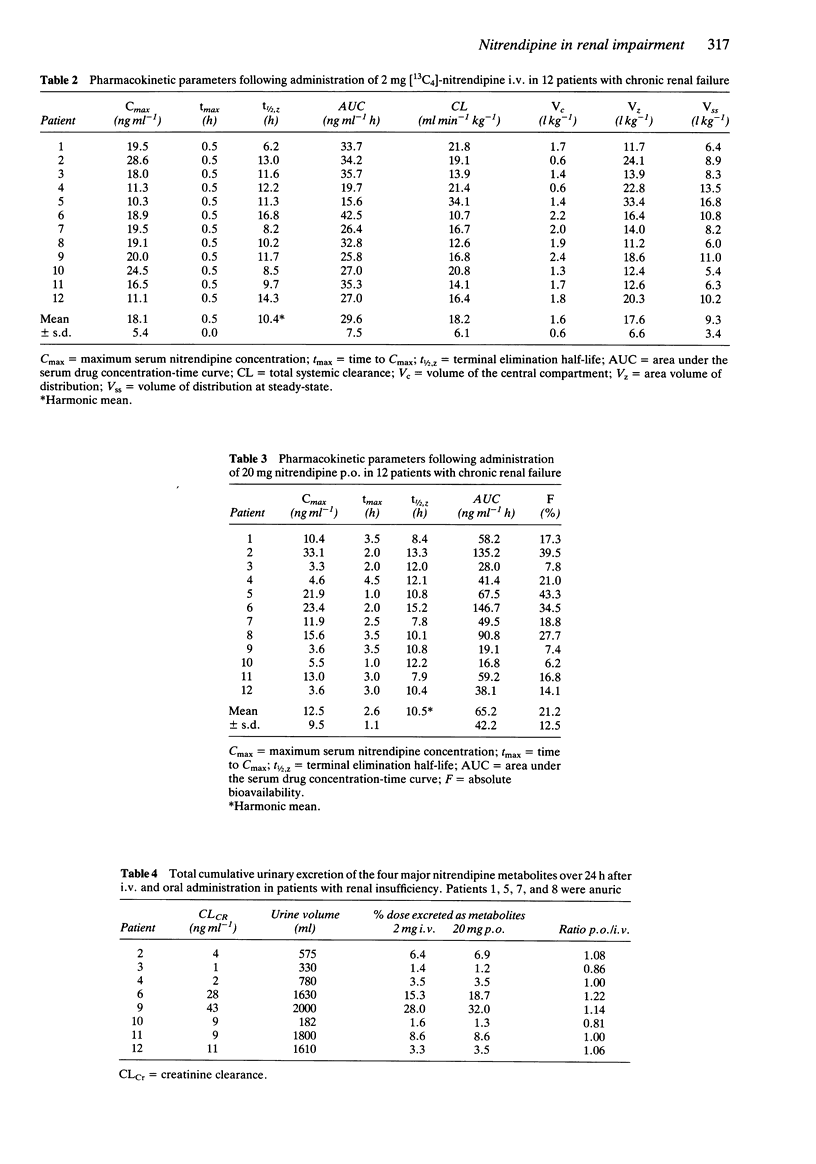
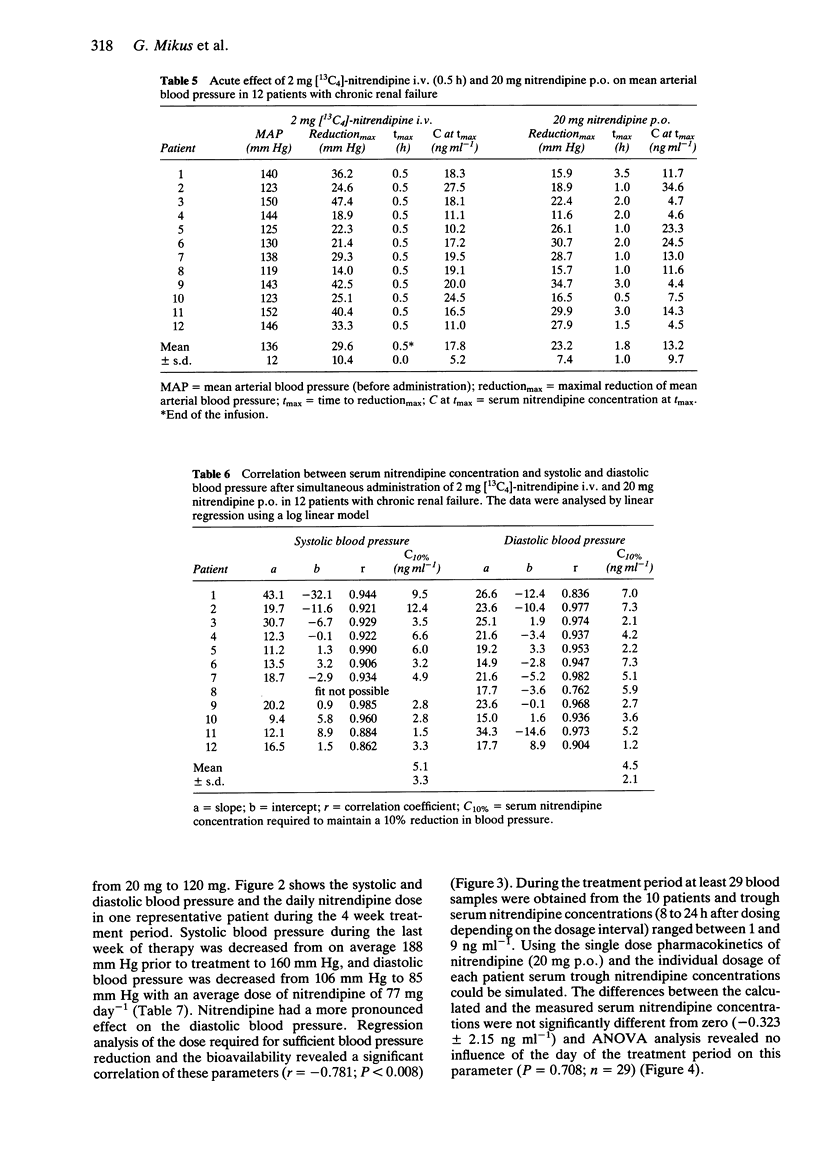
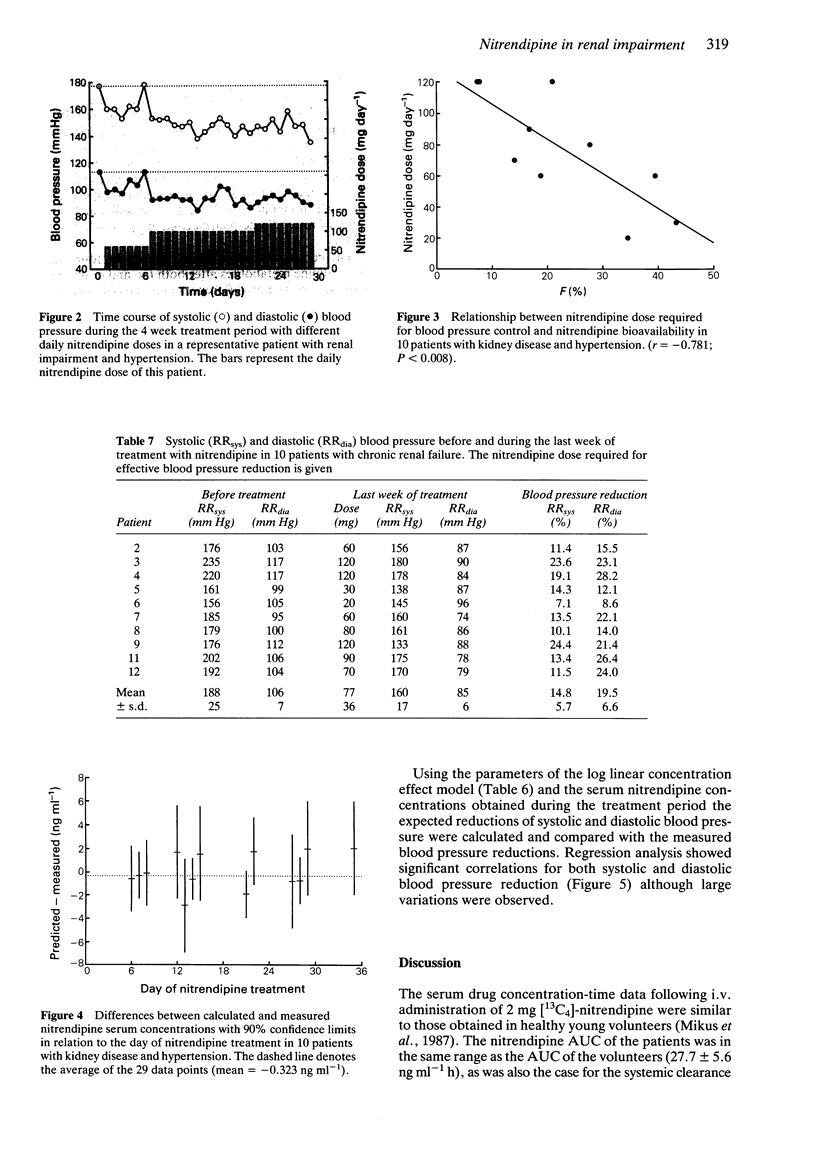
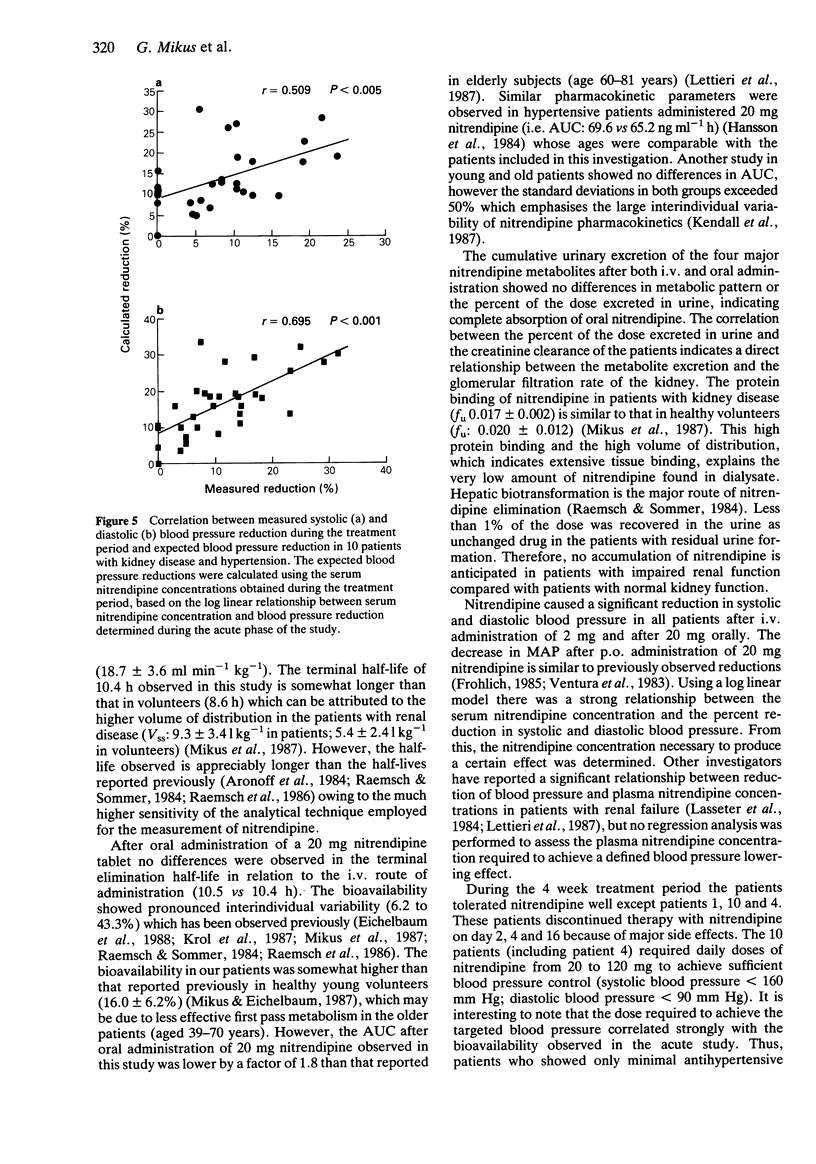
1. The pharmacokinetics, bioavailability, metabolism and antihypertensive effects of nitrendipine have been studied in 12 patients with impaired renal function and moderate to severe hypertension. The drug was administered simultaneously by the i.v. [13C4] and oral (commercial tablet 20 mg) routes. 2. No differences in the pharmacokinetic parameters were observed between the two routes of administration. The systemic clearance after i.v. administration in patients with renal impairment (18.2 +/- 6.1 ml min-1 kg-1) was similar to that observed in healthy volunteers. Despite complete absorption of drug from the tablet the bioavailability of the parent compound was 21.2 +/- 12.5%. Cumulative urinary excretion of nitrendipine metabolites was correlated with the creatinine clearance (r = 0.946). 3. Significant reductions in mean arterial blood pressure (mean: 23.6%) at the end of the nitrendipine infusion and after oral administration of 20 mg (mean: 17.5%) were observed. The blood pressure lowering effect of nitrendipine could be correlated within individuals with serum nitrendipine concentrations using a log linear model. 4. Following 4 weeks of therapy an average dose of 77 mg nitrendipine day-1 was required to achieve a systolic blood pressure below 160 mm Hg or a diastolic blood pressure below 90 mm Hg. The reduction in blood pressure during multiple dosing was related to the nitrendipine steady-state concentration. There was a significant relationship between the nitrendipine bioavailability and the dose required for sufficient blood pressure control. 5. No accumulation of nitrendipine caused by impaired renal function was observed during multiple dosing. Thus, no reduction of the nitrendipine dose in patients with renal impairment is necessary.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankermann T., Osterkamp U., Santos S. R., Kirch W. Elimination and haemodynamic effects of nitrendipine in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(5):433–437. doi: 10.1007/BF00558065. [DOI] [PubMed] [Google Scholar]
- Aronoff G. R., Sloan R. S. Nitrendipine kinetics in normal and impaired renal function. Clin Pharmacol Ther. 1985 Aug;38(2):212–218. doi: 10.1038/clpt.1985.161. [DOI] [PubMed] [Google Scholar]
- Burris J. F., Santangelo R. P., Mroczek W. J. Effectiveness of a new calcium antagonist in severe hypertension. J Clin Pharmacol. 1986 Nov-Dec;26(8):593–597. doi: 10.1002/j.1552-4604.1986.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Dal Canton A., Esposito C., Sabbatini M., Altomonte M., Romano G., Veniero P., Uccello F., Andreucci V. E. Effects of nitrendipine in patients with chronic renal failure. Am J Nephrol. 1986;6 (Suppl 1):162–164. doi: 10.1159/000167242. [DOI] [PubMed] [Google Scholar]
- Fischer C., Heuer B., Heuck K., Eichelbaum M. Quantification of nitrendipine by stable isotope dilution and electron-capture negative ion chemical ionization. Biomed Environ Mass Spectrom. 1986 Dec;13(12):645–650. doi: 10.1002/bms.1200131202. [DOI] [PubMed] [Google Scholar]
- Frohlich E. D. Hemodynamic effects of calcium entry-blocking agents in normal and hypertensive rats and man. Am J Cardiol. 1985 Dec 6;56(16):21H–27H. doi: 10.1016/0002-9149(85)90539-9. [DOI] [PubMed] [Google Scholar]
- Goa K. L., Sorkin E. M. Nitrendipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the treatment of hypertension. Drugs. 1987 Feb;33(2):123–155. doi: 10.2165/00003495-198733020-00003. [DOI] [PubMed] [Google Scholar]
- Kann J. Long-term antihypertensive effects and safety of nitrendipine in patients with mild, moderate, and severe hypertension. J Clin Pharmacol. 1987 Dec;27(12):945–950. doi: 10.1002/j.1552-4604.1987.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Lasseter K. C., Shamblen E. C., Murdoch A. A., Burkholder D. E., Krol G. J., Taylor R. J., Jr, Vanov S. K. Steady-state pharmacokinetics of nitrendipine in hepatic insufficiency. J Cardiovasc Pharmacol. 1984;6 (Suppl 7):S977–S981. [PubMed] [Google Scholar]
- Mikus G., Fischer C., Heuer B., Langen C., Eichelbaum M. Application of stable isotope methodology to study the pharmacokinetics, bioavailability and metabolism of nitrendipine after i.v. and p.o. administration. Br J Clin Pharmacol. 1987 Nov;24(5):561–569. doi: 10.1111/j.1365-2125.1987.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck C. C., Barrett B. B. Nonlinear least-squares regression programs for microcomputers. J Pharmacokinet Biopharm. 1979 Oct;7(5):537–541. doi: 10.1007/BF01062394. [DOI] [PubMed] [Google Scholar]
- Rämsch K. D., Graefe K. H., Scherling D., Sommer J., Ziegler R. Pharmacokinetics and metabolism of calcium-blocking agents nifedipine, nitrendipine, and nimodipine. Am J Nephrol. 1986;6 (Suppl 1):73–80. doi: 10.1159/000167224. [DOI] [PubMed] [Google Scholar]
- Sorkin E. M., Clissold S. P., Brogden R. N. Nifedipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy, in ischaemic heart disease, hypertension and related cardiovascular disorders. Drugs. 1985 Sep;30(3):182–274. doi: 10.2165/00003495-198530030-00002. [DOI] [PubMed] [Google Scholar]
- Ventura H. O., Messerli F. H., Oigman W., Dunn F. G., Reisin E., Frohlich E. D. Immediate hemodynamic effects of a new calcium-channel blocking agent (nitrendipine) in essential hypertension. Am J Cardiol. 1983 Mar 1;51(5):783–786. doi: 10.1016/s0002-9149(83)80133-7. [DOI] [PubMed] [Google Scholar]
- van Bortel L., Böhm R., Mooy J., Schiffers P., Rahn K. H. Pharmacokinetics of nitrendipine in terminal renal failure. Eur J Clin Pharmacol. 1989;36(5):467–471. doi: 10.1007/BF00558071. [DOI] [PubMed] [Google Scholar]


