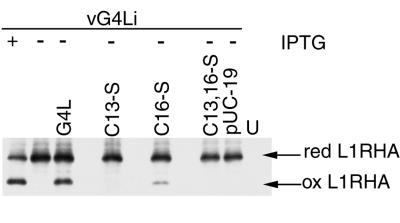
FIG. 5.
Effect of G4L cysteine-to-serine mutations on the formation of disulfide bonds in the L1R protein. BS-C-1 cells were uninfected (U) or infected with vG4Li in the presence (+) or absence (−) of IPTG and cotransfected with plasmids containing HA-tagged L1R and either pUC19, wild-type G4L, or mutated G4L as described in the legend to Fig. 4. At 24 h after infection, proteins were TCA precipitated, solubilized with SDS, alkylated with NEM, and resolved by electrophoresis on a Tris-glycine-10 to 20% polyacrylamide gradient gel. Proteins were detected by Western blotting with MAb HA.11. The reduced (red) and oxidized (ox) forms of L1R are indicated.