Abstract
Swinepox virus (SWPV), the sole member of the Suipoxvirus genus of the Poxviridae, is the etiologic agent of a worldwide disease specific for swine. Here we report the genomic sequence of SWPV. The 146-kbp SWPV genome consists of a central coding region bounded by identical 3.7-kbp inverted terminal repeats and contains 150 putative genes. Comparison of SWPV with chordopoxviruses reveals 146 conserved genes encoding proteins involved in basic replicative functions, viral virulence, host range, and immune evasion. Notably, these include genes with similarity to genes for gamma interferon (IFN-γ) receptor, IFN resistance protein, interleukin-18 binding protein, IFN-α/β binding protein, extracellular enveloped virus host range protein, dUTPase, hydroxysteroid dehydrogenase, superoxide dismutase, serpin, herpesvirus major histocompatibility complex inhibitor, ectromelia virus macrophage host range protein, myxoma virus M011L, variola virus B22R, four ankyrin repeat proteins, three kelch-like proteins, five vaccinia virus (VV) A52R-like family proteins, and two G protein-coupled receptors. The most conserved genomic region is centrally located and corresponds to the VV region located between genes F9L and A38L. Within the terminal 13 kbp, colinearity is disrupted and multiple poxvirus gene homologues are absent or share a lower percentage of amino acid identity. Most of these differences involve genes and gene families with likely functions involving viral virulence and host range. Three open reading frames (SPV018, SPV019. and SPV020) are unique for SWPV. Phylogenetic analysis, genome organization, and amino acid identity indicate that SWPV is most closely related to the capripoxvirus lumpy skin disease virus, followed by the yatapoxvirus yaba-like disease virus and the leporipoxviruses. The gene complement of SWPV better defines Suipoxvirus within the Chordopoxvirinae subfamily and provides a basis for future genetic comparisons.
Swinepox virus (SWPV) is the sole member of the Suipoxvirus genus, one of eight genera within the Chordopoxvirinae subfamily of the Poxviridae. It is responsible for swinepox, a disease that occurs worldwide and is associated with poor sanitation (18).
Swinepox is most severe in young pigs (up to 4 months of age), where morbidity may approach 100% (18, 30). Adults generally develop a mild, self-limiting form of the disease, with lesions in hairless skin areas that remain localized at the sites of entry (7, 18, 30). In adults, macroscopic cutaneous lesions pass through the characteristic stages of poxviral lesions with a very short vesicular phase that usually does not exhibit fluid exudates (9, 18, 30). SWPV infection in swine is characterized by slight fever and inflammation of local lymph nodes. Generalized infection and viremia are not observed (22). The source and the reservoir of SWPV are infected swine (18). Lice are considered the primary agents of transmission, but occasional horizontal transmission may occur through contact of nasal and oral secretions with skin abrasions (18).
SWPV infects only swine. Of several mammalian and avian species tested, only rabbits produced a nonproductive infection after intradermal inoculation (8). The host range specificity of SWPV and its ability to induce solid protective immunity have stimulated interest in using SWPV as a host range-restricted vaccine vector (14, 41, 45).
Current molecular data describing the SWPV genome consist of restriction endonuclease analysis and limited DNA sequence analysis of the terminal regions (4, 12, 25, 26, 34). Given the interest in developing more effective SWPV vaccines and expression vectors, we have sequenced and analyzed the genome of a pathogenic SWPV. These data provide a comprehensive view of the SWPV genome and further elucidate the relationship of SWPV to other chordopoxviruses (ChPVs). Furthermore, they define the gene complement that underlies the virulence and restricted host range of SWPV.
MATERIALS AND METHODS
SWPV DNA isolation, cloning, sequencing, and sequence analysis.
SWPV genomic DNA was extracted from pig kidney cells infected with virus obtained from a pig litter congenitally infected during an outbreak of swinepox in Nebraska in 1999. These animals displayed foci of full-thickness epithelial necrosis distributed over the entire skin and tongue. Random DNA fragments were obtained by incomplete enzymatic digestion with Tsp509I endonuclease (New England Biolabs, Beverly, Mass.), and DNA fragments of 1.0 to 6.0 kbp were cloned and sequenced as previously described (2). Reaction products were run on a PRISM 3700 automated DNA sequencer (PE Biosystems, Foster City, Calif.). Sequence data were assembled with the Phrap software program and gaps were closed as described previously (1, 11), with confirmatory assemblies performed using CAP3 (19). The final DNA consensus sequence represented on average eightfold redundancy at each base position.
Genome DNA composition, structure, repeats, and restriction enzyme patterns were analyzed as previously described (1) using the Genetics Computer Group version 10 software package (10). Open reading frames (ORFs) longer than 30 codons were evaluated for coding potential as previously described (2). All potentially coding ORFs and ORFs greater than 60 codons were evaluated by homology searches as previously described (1, 2). Using these criteria, 150 ORFs were annotated as potential genes. Gene family regions were analyzed with Geanfammer (32) and promoters were analyzed with Genetics Computer Group MEME programs (10), and annotation was as previously described (1, 2). The vaccinia virus (VV) A52R-like protein family was clustered from a nonredundant peptide database of all known poxvirus sequences using the CLUS program (23) and BLASTP2 scores of greater than 115. Phylogenetic comparisons were done with the PHYLO_WIN software package (15).
Nucleotide sequence accession number.
The SWPV genome sequence was assigned GenBank accession no. AF410153.
RESULTS AND DISCUSSION
Organization of the SWPV genome.
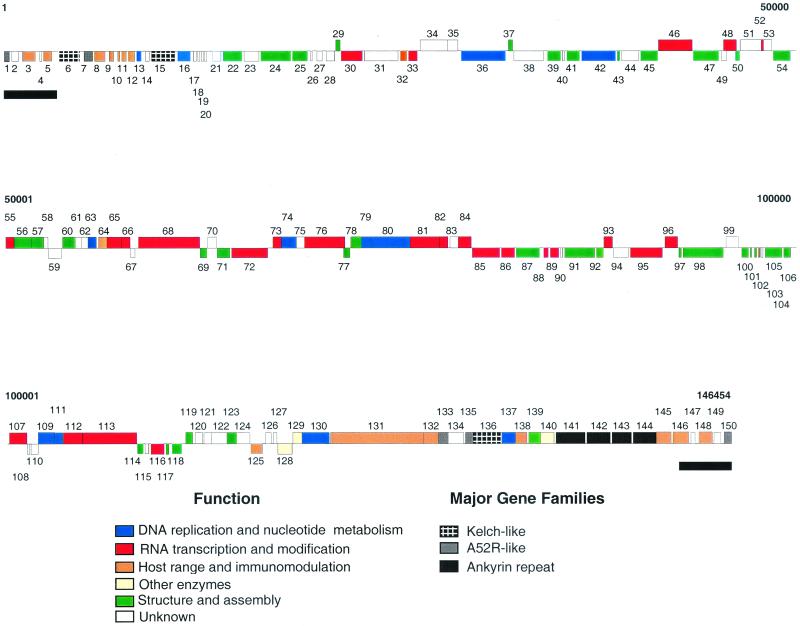
SWPV genome sequences were assembled into a contiguous sequence of 146,454 bp, which agrees with a previous restriction enzyme-based size estimate of 148 to 150 kbp and previously published restriction enzyme maps (26). Because the hairpin loops were not sequenced, the leftmost nucleotide was arbitrarily designated base 1. The nucleotide composition is 72.5% A+T and is uniformly distributed. As found in other poxviruses, the SWPV genome contains a 139,023-bp central coding region bounded by two identical inverted terminal repeat (ITR) regions of 3.7 kbp (Fig. 1). The most-terminal nucleotides of the assembled sequence contain a 34-bp perfect tandem repeat which is part of a 65-bp imperfect repeat. Based on these data and published restriction digests, we estimated that heterogeneity of termini containing repeats may account for an additional 200 to 1,200 bp (26).
FIG. 1.
Linear map of the SWPV genome. ORFs are numbered from left to right based on the position of the methionine initiation codon. ORFs transcribed to the right are located above the horizontal line; ORFs transcribed to the left are below. Genes with similar functions and members of gene families are colored as indicated. ITRs are represented as black bars below the ORF map.
SWPV has a compact gene arrangement with almost no overlapping ORFs and no evidence of introns or large regions of noncoding DNA. SWPV contains 150 putative genes which encode proteins of 53 to 1,959 amino acids, of which 146 are poxvirus homologues (Fig. 1; Table 1). The conserved central core (SPV021 to SPV125), which is colinear with VV F9L to A38L, contains 106 genes, most of which are involved in basic replicative functions. In terminal genomic regions, genes are oriented toward the ends, while in the central region, genes are oriented in both directions, often grouped in clusters with identical orientation. SWPV promoters resemble other ChPV promoters (Table 1). As is found in other poxviruses, many of the 30 putative early genes are members of gene families and putative host range genes, while the 45 genes containing the VV late promoter sequence (TAAATG) at the ATG codon (28) encode many of the conserved virion-associated poxviral proteins (Table 1).
TABLE 1.
SWPV ORFs
| ORF | Position (length, codons) | SPV accession no.a | Best match
|
Predicted structure and/or functiond | Promoter typee | LSDV
|
YLDV
|
MYX
|
VV
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Accession no.a | BLASTP2 score | % Id.j | Length (aa)c | ORFf | % Id.j | ORFg | % Id.j | ORFh | % Id.j | ORFi | % Id.j | |||||
| SPV001 | 736-287 (150) | LSDV | AF325528 | 498 | 62 | 145 | A52R family protein | LSDV001 | 62 | 6L | 41 | M003.1 | 45 | B15R | 41 | ||
| SPV002 | 1323-814 (170) | ShPVb | P18388 | 195 | 39 | 113 | TM | LSDV002 | 39 | M003.2 | 29 | ||||||
| SPV003 | 2452-1433 (340) | P32231 | YLDV | AJ293568 | 539 | 38 | 335 | MHC class 1 α chain-like protein, TM | E | 2L | 38 | ||||||
| SPV004 | 2772-2497 (92) | P32230 | LSDV | AF325528 | 217 | 46 | 91 | LSDV004 | 46 | 150R | 42 | M004.1 | 44 | ||||
| SPV005 | 3630-2824 (269) | Q08520 | Human | U45983 | 497 | 37 | 267 | GPCR, TM | LSDV011 | 38 | 7L/145R | 36 | |||||
| SPV006 | 5285-3696 (530) | P32228 | RFV | AF170722 | 824 | 33 | 523 | Kelch-like protein | LSDV151 | 27 | 140R | 28 | M008L | 34 | A55R | 29 | |
| SPV007 | 6038-5331 (236) | P32227 | YLDV | AJ293568 | 444 | 43 | 217 | A52R family protein | LSDV009 | 29 | 3L | 43 | K7R | 35 | |||
| SPV008 | 6885-6064 (274) | P32226 | LSDV | AF325528 | 435 | 37 | 257 | IFN-γ receptor, SP, TM | E | LSDV008 | 37 | M007 | 25 | B8R | 28 | ||
| SPV009 | 7385-6921 (155) | P32225 | LSDV | AF325528 | 309 | 35 | 150 | LAP/PHD-finger protein, TM | LSDV010 | 35 | 5L | 34 | M153R | 36 | |||
| SPV010 | 7705-7448 (86) | P32224 | LSDV | AF325528 | 248 | 49 | 85 | elF2α-like PKR inhibitor | E | LSDV014 | 49 | 12L | 48 | M156R | 40 | K3L | 44 |
| SPV011 | 8146-7745 (134) | P32223 | LSDV | AF325528 | 308 | 44 | 129 | IL-18 binding protein | LSDV015 | 44 | 14L | 42 | |||||
| SPV012 | 8672-8172 (167) | P32222 | LSDV | AF325528 | 168 | 34 | 171 | Integral membrane protein, apoptosis regulator | E | LSDV017 | 34 | 16L | 31 | M011L | 27 | ||
| SPV013 | 9153-8728 (142) | P32208 | LSDV | AF325528 | 505 | 68 | 141 | dUTPase | LSDV018 | 68 | 17L | 65 | M012L | 59 | F2L | 58 | |
| SPV014 | 9576-9205 (124) | P32221 | YLDV | AJ293568 | 243 | 38 | 124 | E | 18L | 38 | M013L | 36 | |||||
| SPV015 | 11205-9604 (534) | P32206 | LSDV | AF325528 | 1,100 | 40 | 564 | Kelch-like protein | LSDV019 | 40 | 19L | 38 | M014L | 39 | F3L | 28 | |
| SPV016 | 12243-11284 (320) | P32209 | VAR | U18338 | 1,341 | 77 | 320 | Ribonucleotide reductase (small subunit) TM | E | LSDV020 | 78 | 20L | 78 | M015L | 74 | F4L | 76 |
| SPV017 | 12529-12272 (86) | P32220 | LSDV | AF325528 | 179 | 38 | 86 | SP, TM | LSDV021 | 38 | 21L | 33 | M016L | 28 | |||
| SPV018 | 12789-12571 (73) | P32219 | |||||||||||||||
| SPV019 | 13012-12803 (70) | P32218 | E | ||||||||||||||
| SPV020 | 13142-12942 (67) | P32217 | SP, TM | ||||||||||||||
| SPV021 | 14156-13512 (215) | P32207 | LSDV | AF325528 | 754 | 64 | 216 | TM | L | LSDV024 | 64 | 24L | 50 | M019L | 52 | F9L | 45 |
| SPV022 | 15462-14143 (440) | P32216 | LSDV | AF325528 | 1,996 | 80 | 435 | Ser/Thr protein kinase, virus assembly | L | LSDV025 | 80 | 25L | 76 | M020L | 77 | F10L | 72 |
| SPV023 | 16562-15486 (359) | VV | AF095689 | 448 | 31 | 351 | E | LSDV026 | 34 | F11L | 31 | ||||||
| SPV024 | 18530-16596 (645) | LSDV | AF325528 | 1,546 | 51 | 631 | EEV maturation, TM | E | LSDV027 | 51 | A26L | 49 | M021L | 46 | F12L | 39 | |
| SPV025 | 19678-18566 (371) | AJ249689 | ShPV | AF199594 | 1,499 | 76 | 368 | EEV envelope protein, virus assembly | LSDV028 | 75 | 27L | 74 | M022L | 73 | F13L | 56 | |
| SPV026 | 19915-19727 (63) | AL109739 | 72 | 30 | 53 | ||||||||||||
| SPV027 | 20589-20146 (148) | LSDV | AF325528 | 514 | 66 | 148 | LSDV029 | 66 | 29L | 62 | M024L | 49 | F15L | 60 | |||
| SPV028 | 21311-20661 (217) | LSDV | AF325528 | 502 | 44 | 218 | LSDV030 | 44 | 30L | 39 | M025L | 36 | F16L | 42 | |||
| SPV029 | 21372-21680 (103) | YLDV | AJ293568 | 388 | 72 | 100 | DNA binding virion core phosphoprotein | L | LSDV031 | 71 | 31R | 72 | M026L | 70 | F17R | 62 | |
| SPV030 | 23092-21683 (470) | LSDV | AF325528 | 1,877 | 75 | 474 | Poly(A) polymerase PAP | LSDV032 | 75 | 32L | 71 | M027L | 74 | E1L | 65 | ||
| SPV031 | 25324-23129 (732) | LSDV | AF325528 | 2,089 | 55 | 714 | LSDV033 | 55 | 33L | 50 | M028L | 53 | E2L | 44 | |||
| SPV032 | 25909-25379 (177) | YLDV | AJ293568 | 471 | 50 | 180 | PKR inhibitor, host range | E | LSDV034 | 49 | 34L | 50 | M029L | 50 | E3L | 34 | |
| SPV033 | 26552-25938 (205) | LSDV | AF325528 | 795 | 71 | 199 | RNA polymerase subunit RPO30 | E | LSDV036 | 71 | 35L | 70 | M030R | 68 | E4L | 68 | |
| SPV034 | 26660-28363 (568) | LSDV | AF325528 | 2,267 | 73 | 568 | E/L | LSDV037 | 73 | 37R | 68 | M032R | 67 | E6R | 60 | ||
| SPV035 | 28370-29155 (262) | LSDV | AF325528 | 1,147 | 80 | 265 | ER-localized protein, TM | LSDV038 | 80 | 38R | 74 | M033R | 81 | E8R | 66 | ||
| SPV036 | 32190-29167 (1008) | MYX | AF170726 | 4,123 | 75 | 1,008 | DNA polymerase | E | LSDV039 | 75 | 39L | 68 | M034L | 75 | E9L | 66 | |
| SPV037 | 32223-32510 (96) | MYX | AF170726 | 382 | 67 | 96 | Potential redox protein, virus assembly | LSDV040 | 69 | 40R | 71 | M035R | 67 | E10R | 66 | ||
| SPV038 | 34587-32557 (677) | MYX | AF170726 | 1,521 | 42 | 678 | E | LSDV042 | 43 | 42L | 43 | M036L | 42 | O1L | 34 | ||
| SPV039 | 35664-34726 (313) | LSDV | AF325528 | 1,187 | 72 | 314 | DNA binding virion core protein, virus assembly | L | LSDV043 | 72 | 43L | 67 | M038L | 70 | I1L | 66 | |
| SPV040 | 35892-35668 (75) | RFV | AF170722 | 202 | 53 | 73 | TM | L | LSDV044 | 52 | 44L | 53 | M039L | 57 | 12L | 59 | |
| SPV041 | 36756-35896 (287) | LSDV | AF325528 | 915 | 65 | 276 | DNA binding phosphoprotein | LSDV045 | 65 | 45L | 61 | M040L | 60 | I3L | 53 | ||
| SPV042 | 39041-36771 (757) | VAR | P32984 | 2,727 | 66 | 751 | Ribonucleotide reductase (large chain) | I4L | 66 | ||||||||
| SPV043 | 39324-39091 (78) | LSDV | AF325528 | 264 | 56 | 78 | IMV membrane protein, SP, TM | L | LSDV046 | 56 | 46L | 55 | M041L | 51 | I5L | 31 | |
| SPV044 | 40497-39343 (385) | LSDV | AF325528 | 1,181 | 57 | 394 | TM | LSDV047 | 57 | 47L | 50 | M042L | 52 | I6L | 51 | ||
| SPV045 | 41791-40493 (433) | LSDV | AF325528 | 1,792 | 75 | 433 | Virion core protein | L | LSDV048 | 75 | 48L | 74 | M043L | 74 | I7L | 68 | |
| SPV046 | 41797-43839 (681) | LSDV | AF325528 | 2,315 | 64 | 681 | NPH-II, RNA helicase | LSDV049 | 64 | 49R | 61 | M044R | 62 | I8R | 58 | ||
| SPV047 | 45620-43842 (593) | LSDV | AF325528 | 2,158 | 67 | 595 | Metalloprotease, virion morphogenesis | L | LSDV050 | 67 | 50L | 63 | M045L | 64 | G1L | 57 | |
| SPV048 | 45946-46641 (232) | LSDV | AF325528 | 768 | 63 | 222 | Putative transcriptional elongation factor | LSDV051 | 63 | 52R | 48 | M047R | 55 | G2R | 44 | ||
| SPV049 | 45952-45620 (111) | RFV | AF170722 | 384 | 63 | 111 | TM | L | LSDV052 | 58 | 51L | 54 | M046L | 63 | G3L | 47 | |
| SPV050 | 46958-46584 (125) | LSDV | AF325528 | 488 | 70 | 124 | Glutaredoxin 2, virion morphogenesis, | L | LSDV053 | 70 | 53L | 64 | M048L | 64 | G4L | 43 | |
| SPV051 | 46963-48279 (439) | LSDV | AF325528 | 1,486 | 63 | 438 | LSDV054 | 63 | 54R | 54 | M049R | 52 | G5R | 46 | |||
| SPV052 | 48285-48473 (63) | LSDV | AF325528 | 286 | 87 | 63 | RNA polymerase subunit RPO7 | E | LSDV055 | 87 | 55R | 84 | M050R | 82 | G5.5R | 77 | |
| SPV053 | 48476-48994 (173) | LSDV | AF325528 | 539 | 60 | 169 | TM | LSDV056 | 60 | 56R | 55 | M051R | 59 | G6R | 43 | ||
| SPV054 | 50144-49002 (381) | LSDV | AF325528 | 1,227 | 65 | 378 | Virion core protein, TM | L | LSDV057 | 65 | 57L | 60 | M052L | 54 | G7L | 53 | |
| SPV055 | 50174-50953 (260) | LSDV | AF325528 | 1,245 | 91 | 260 | Late transcription factor VLTF-1, TM | LSDV058 | 91 | 58R | 88 | M053R | 85 | G8R | 83 | ||
| SPV056 | 50949-52016 (356) | LSDV | AF325528 | 1,038 | 58 | 336 | Myristylated protein | LSDV059 | 58 | 59R | 56 | M054R | 52 | G9R | 45 | ||
| SPV057 | 52020-52766 (249) | LSDV | AF325528 | 1,111 | 85 | 245 | Myristylated IMV envelope protein, TM | L | LSDV060 | 85 | 60R | 81 | M055R | 75 | L1R | 67 | |
| SPV058 | 52799-53080 (94) | LSDV | AF325528 | 242 | 50 | 85 | TM | E | LSDV061 | 50 | 61R | 40 | M056R | 29 | L2R | 31 | |
| SPV059 | 54025-53066 (320) | LSDV | AF325528 | 1,205 | 70 | 320 | L | LSDV062 | 70 | 62L | 65 | M057R | 65 | L3L | 49 | ||
| SPV060 | 54051-54809 (253) | LSDV | AF325528 | 1,078 | 81 | 253 | DNA-binding virion core protein VP8 | L | LSDV063 | 81 | 63R | 77 | M058R | 79 | L4R | 62 | |
| SPV061 | 54824-55210 (129) | LSDV | AF325528 | 412 | 64 | 126 | TM | L | LSDV064 | 64 | 64R | 54 | M059R | 52 | L5R | 50 | |
| SPV062 | 55167-55610 (148) | P23332 | ShPV | P19746 | 451 | 60 | 148 | L | LSDV065 | 60 | 65R | 57 | M060R | 53 | J1R | 53 | |
| SPV063 | 55625-56167 (181) | M64000 | ShPV | P16600 | 640 | 68 | 175 | Thymidine kinase, SP | LSDV066 | 68 | 66R | 66 | M061R | 67 | J2R | 64 | |
| SPV064 | 56225-56779 (185) | P23333 | ShPV | P19747 | 467 | 48 | 187 | Host range protein | E | LSDV067 | 48 | 67R | 45 | M062R | 41 | C7L | 39 |
| SPV065 | 56835-57833 (333) | RFV | AF170722 | 1,430 | 79 | 333 | Poly(A) polymerase PAPs | LSDV068 | 77 | 68R | 72 | M065R | 78 | J3R | 72 | ||
| SPV066 | 57751-58305 (185) | LSDV | AF325528 | 816 | 81 | 185 | RNA polymerase subunit RPO22 | LSDV069 | 81 | 69R | 75 | M066R | 72 | J4R | 66 | ||
| SPV067 | 58715-58314 (134) | RFV | AF170722 | 541 | 68 | 132 | LSDV070 | 67 | 70L | 65 | M067L | 67 | J5L | 60 | |||
| SPV068 | 58782-62636 (1285) | LSDV | AF325528 | 5,865 | 84 | 1,285 | RNA polymerase subunit RPO147 | LSDV071 | 84 | 71R | 84 | M068R | 84 | J6R | 80 | ||
| SPV069 | 63169-62651 (173) | ShPV | AF124517 | 718 | 78 | 171 | Protein tyrosine phosphatase, virus assembly | L | LSDV072 | 78 | 72L | 72 | M069L | 73 | H1L | 61 | |
| SPV070 | 63184-63753 (190) | ShPV | AF124517 | 789 | 73 | 190 | TM | LSDV073 | 73 | 73R | 67 | M070R | 71 | H2R | 65 | ||
| SPV071 | 64742-63771 (324) | LSDV | AF325528 | 1,075 | 61 | 320 | IMV envelope protein p35, TM | L | LSDV074 | 61 | 74L | 54 | M071L | 54 | H3L | 40 | |
| SPV072 | 67148-64746 (801) | LSDV | AF325528 | 3,378 | 78 | 801 | RNA polymerase-associated protein RAP94 | L | LSDV075 | 78 | 75L | 76 | M072L | 75 | H4L | 69 | |
| SPV073 | 67315-67857 (181) | YLDV | AJ293568 | 436 | 50 | 181 | Late transcription factor VLTF-4 | E | LSDV076 | 46 | 76R | 50 | M073R | 48 | H5R | 37 | |
| SPV074 | 67896-68855 (320) | LSDV | AF325528 | 1,204 | 72 | 315 | DNA topoisomerase | LSDV077 | 72 | 77R | 61 | M074R | 66 | H6R | 61 | ||
| SPV075 | 68867-69313 (149) | LSDV | AF325528 | 499 | 68 | 145 | L | LSDV078 | 68 | 78R | 53 | M075R | 55 | H7R | 43 | ||
| SPV076 | 69320-71839 (840) | LSDV | AF325528 | 3,320 | 72 | 842 | mRNA capping enzyme (large subunit) | LSDV079 | 72 | 79R | 70 | M076R | 71 | D1R | 65 | ||
| SPV077 | 72244-71807 (146) | YLDV | AJ293568 | 355 | 47 | 149 | Virion protein | LSDV080 | 46 | 80L | 47 | M077L | 46 | D2L | 40 | ||
| SPV078 | 72249-72980 (244) | LSDV | AF325528 | 469 | 39 | 241 | Virion protein | LSDV081 | 39 | 81R | 37 | M078R | 37 | D3R | 34 | ||
| SPV079 | 72980-73630 (217) | RFV | AF170126 | 926 | 72 | 216 | Uracil DNA glycosylase, DNA replication | LSDV082 | 73 | 82R | 73 | M079R | 72 | D4R | 69 | ||
| SPV080 | 73663-76020 (786) | LSDV | AF325528 | 3,324 | 76 | 786 | NTPase, DNA replication, TM | L | LSDV083 | 76 | 83R | 77 | M080R | 76 | D5R | 68 | |
| SPV081 | 76020-77924 (635) | LSDV | AF325528 | 3,004 | 90 | 635 | Early transcription factor VETFs, TM | L | LSDV084 | 90 | 84R | 87 | M081R | 87 | D6R | 81 | |
| SPV082 | 77952-78434 (161) | LSDV | AF325528 | 731 | 81 | 160 | RNA polymerase subunit RPO18 | L | LSDV085 | 81 | 85R | 75 | M082R | 77 | D7R | 63 | |
| SPV083 | 78507-79145 (213) | LSDV | AF325528 | 744 | 69 | 213 | mutT motif | E | LSDV086 | 69 | 86R | 64 | M084R | 61 | D9R | 57 | |
| SPV084 | 79145-79870 (242) | RFV | P32097 | 709 | 56 | 237 | mutT motif, gene expression regulator | L | LSDV087 | 55 | 87R | 61 | M085R | 59 | D10R | 45 | |
| SPV085 | 81766-79871 (632) | LSDV | AF325528 | 2,606 | 77 | 632 | NPH-I, transcription termination factor | L | LSDV088 | 77 | 88L | 73 | M086L | 74 | D11L | 69 | |
| SPV086 | 82647-81787 (287) | Q08512 | LSDV | AF325528 | 1,259 | 80 | 287 | mRNA capping enzyme (small subunit), VITF | E/L | LSDV089 | 80 | 89L | 82 | M087L | 76 | D12L | 77 |
| SPV087 | 84330-82678 (551) | Q08517 | YLDV | AJ293568 | 2,347 | 78 | 550 | Rifampin resistance protein, IMV assembly | LSDV090 | 77 | 90L | 78 | M088L | 76 | D13L | 70 | |
| SPV088 | 84807-84358 (150) | LSDV | AF325528 | 529 | 65 | 150 | Late transcription factor VLTF-2 | L | LSDV091 | 65 | 91L | 62 | M089L | 64 | A1L | 57 | |
| SPV089 | 85531-84860 (224) | LSDV | AF325528 | 1,039 | 87 | 225 | Late transcription factor VLTF-3 | LSDV092 | 87 | 92L | 84 | M090L | 85 | A2L | 80 | ||
| SPV090 | 85755-85531 (75) | LSDV | AF325528 | 297 | 72 | 75 | L | LSDV093 | 72 | 93L | 56 | M091L | 66 | 8.9 kDa* | 50 | ||
| SPV091 | 87720-85765 (652) | LSDV | AF325528 | 2,736 | 78 | 656 | Virion core protein P4b | L | LSDV094 | 78 | 94L | 78 | M092L | 75 | A3L | 67 | |
| SPV092 | 88230-87769 (154) | LSDV | AF325528 | 407 | 52 | 158 | Virion core protein, virion morphogenesis | L | LSDV095 | 52 | 95L | 38 | M093L | 32 | A4L | 27 | |
| SPV093 | 88271-88756 (162) | LSDV | AF325528 | 542 | 65 | 170 | RNA polymerase subunit RPO19 | L | LSDV096 | 65 | 96R | 57 | M094R | 65 | A5R | 59 | |
| SPV094 | 89877-88759 (373) | MYX | AF170726 | 1,529 | 77 | 372 | LSDV097 | 78 | 97L | 70 | M095L | 77 | A6L | 56 | |||
| SPV095 | 92035-89903 (711) | LSDV | AF325528 | 3,027 | 80 | 714 | Early transcription factor VETFL | LSDV098 | 80 | 98L | 77 | M096L | 80 | A7L | 71 | ||
| SPV096 | 92097-92972 (292) | LSDV | AF325528 | 1,090 | 74 | 288 | Intermediate transcription factor VITF-3 | LSDV099 | 74 | 99R | 68 | M097R | 70 | A8R | 62 | ||
| SPV097 | 93226-92975 (84) | LSDV | AF325528 | 352 | 84 | 78 | IMV membrane protein, morphogenesis, SP, TM | L | LSDV100 | 84 | 100L | 83 | M098L | 80 | A9L | 69 | |
| SPV098 | 95941-93230 (904) | LSDV | AF325528 | 3,491 | 72 | 904 | Virion core protein P4a | L | LSDV101 | 72 | 101L | 68 | M099L | 65 | A10L | 51 | |
| SPV099 | 95956-96888 (311) | YMTV | AB015885 | 1,237 | 76 | 314 | L | LSDV102 | 75 | 102R | 77 | M100R | 75 | A11R | 53 | ||
| SPV100 | 97433-96900 (178) | MYX | AF170726 | 435 | 66 | 153 | Virion core protein | L | LSDV103 | 57 | 103L | 62 | M101L | 66 | A12L | 52 | |
| SPV101 | 97657-97454 (68) | LSDV | AF325528 | 190 | 57 | 68 | IMV membrane protein, TM | L | LSDV104 | 57 | 104L | 54 | M102L | 48 | A13L | 33 | |
| SPV102 | 98006-97725 (94) | YLDV | AJ293568 | 420 | 87 | 94 | IMV membrane protein, SP, TM | L | LSDV105 | 82 | 105L | 87 | M103L | 70 | A14L | 55 | |
| SPV103 | 98184-98026 (53) | YLDV | AJ293568 | 235 | 84 | 52 | Virulence factor, SP | LSDV106 | 75 | 106L | 84 | M104L | 76 | A14.5 | 56 | ||
| SPV104 | 98458-98177 (94) | YMTV | AB015885 | 263 | 51 | 94 | L | LSDV107 | 49 | 107L | 46 | M105L | 50 | A15L | 47 | ||
| SPV105 | 99581-98445 (379) | LSDV | AF325528 | 1,387 | 66 | 379 | Putative myristylated membrane protein, TM | L | LSDV108 | 66 | 108L | 60 | M106L | 60 | A16L | 52 | |
| SPV106 | 100192-99611 (194) | YLDV | AJ293568 | 692 | 69 | 194 | Phosphorylated IMV membrane protein, TM | L | LSDV109 | 64 | 109L | 69 | M107L | 54 | A17L | 42 | |
| SPV107 | 100207-101643 (479) | LSDV | AF325528 | 1,623 | 65 | 480 | DNA helicase, transcriptional elongation, TM | LSDV110 | 65 | 110R | 63 | M108R | 61 | A18R | 57 | ||
| SPV108 | 101845-101630 (72) | LSDV | AF325528 | 292 | 76 | 72 | L | LSDV111 | 76 | 111L | 72 | M109L | 76 | A19L | 63 | ||
| SPV109 | 102189-103472 (428) | LSDV | AF325528 | 1,266 | 57 | 427 | DNA polymerase proces- sivity factor | E | LSDV112 | 57 | 113R | 48 | M111R | 54 | A20R | 46 | |
| SPV110 | 102190-101849 (114) | MYX | AF170726 | 391 | 64 | 114 | TM | LSDV113 | 62 | 112L | 62 | M110L | 64 | A21L | 59 | ||
| SPV111 | 103453-103950 (166) | VAR | X76268 | 653 | 71 | 164 | DNA processing | E | LSDV114 | 64 | 114R | 71 | M112R | 61 | A22R | 71 | |
| SPV112 | 103979-105124 (382) | LSDV | AF325528 | 1,274 | 66 | 383 | Intermediate transcription factor VITF-3 | LSDV115 | 66 | 115R | 63 | M113R | 62 | A23R | 59 | ||
| SPV113 | 105129-108614 (1162) | LSDV | AF325528 | 5,459 | 89 | 1,156 | RNA polymerase subunit RPO132 | LSDV116 | 89 | 116R | 87 | M114R | 86 | A24R | 82 | ||
| SPV114 | 109061-108615 (149) | LSDV | AF325528 | 345 | 48 | 144 | Fusion protein, virus assembly | L | LSDV117 | 48 | 117L | 41 | M115L | 52 | A27L | 45 | |
| SPV115 | 109485-109054 (144) | ShPV | P16718 | 539 | 71 | 139 | SP, TM | L | LSDV118 | 71 | 118L | 64 | M116L | 65 | A28L | 50 | |
| SPV116 | 110404-109499 (302) | MYX | AF170726 | 1,137 | 65 | 302 | RNA polymerase subunit RPO35 | E | LSDV119 | 68 | 119L | 63 | M117L | 65 | A29L | 59 | |
| SPV117 | 110597-110376 (74) | YLDV | AJ293568 | 220 | 63 | 73 | Virion protein, maturation | LSDV120 | 61 | 120L | 63 | M118L | 57 | A30L | 51 | ||
| SPV118 | 111542-110787 (252) | LSDV | AF325528 | 1,109 | 82 | 252 | DNA packaging, virus assembly | LSDV121 | 82 | 121L | 79 | M120L | 80 | A32L | 58 | ||
| SPV119 | 111706-112260 (185) | LSDV | AF325528 | 356 | 44 | 179 | EEV glycoprotein, TM | LSDV122 | 44 | 122R | 41 | M121R | 38 | A33R | 30 | ||
| SPV120 | 112290-112796 (169) | YLDV | AJ293568 | 644 | 64 | 169 | EEV protein | LSDV123 | 58 | 123R | 64 | M122R | 60 | A34R | 50 | ||
| SPV121 | 112809-113363 (185) | LSDV | AF325528 | 444 | 45 | 177 | E | LSDV124 | 45 | 124R | 46 | M123R | 39 | A35R | 36 | ||
| SPV122 | 113341-114282 (314) | LSDV | AF325528 | 785 | 53 | 288 | TM | LSDV125 | 53 | 125R | 39 | M124R | 44 | ||||
| SPV123 | 114313-114909 (199) | YLDV | AJ293568 | 244 | 40 | 191 | EEV glycoprotein, TM | LSDV126 | 36 | 126R | 40 | M125R | 28 | A36R | 25 | ||
| SPV124 | 114913-115752 (280) | LSDV | AF325528 | 752 | 51 | 271 | TM | L | LSDV127 | 51 | 127R | 40 | M126R | 44 | A37R | 28 | |
| SPV125 | 116661-115783 (293) | MYX | AF170726 | 576 | 38 | 281 | CD47-like protein, SP, TM | LSDV128 | 31 | 128L | 30 | M128L | 38 | A38L | 21 | ||
| SPV126 | 116801-117178 (126) | LSDV | AF325528 | 128 | 33 | 112 | LSDV129 | 33 | M130R | 28 | |||||||
| SPV127 | 117250-117486 (79a) | LSDV | AF325528 | 180 | 54 | 75 | LSDV130 | 54 | 132R | 40 | |||||||
| SPV128 | 118520-117489 (344) | YLDV | AJ293568 | 959 | 53 | 343 | Hydroxysteroid dehydrogenase-like protein | E | 133L | 53 | A44L | 45 | |||||
| SPV129 | 118565-119053 (163) | LSDV | AF325528 | 550 | 64 | 159 | Superoxide dismutase-like protein | LSDV131 | 64 | M131R | 59 | A45R | 31 | ||||
| SPV130 | 119089-120765 (559) | LSDV | AF325528 | 1,912 | 63 | 558 | DNA ligase | LSDV133 | 63 | M133R | 61 | A50R | 50 | ||||
| SPV131 | 120870-126746 (1959) | LSDV | AF325528 | 5,419 | 56 | 2,007 | VAR B22R homologue, TM | LSDV134 | 56 | 135R | 50 | M134R | 50 | ||||
| SPV132 | 126727-127758 (344) | YLDV | AJ293568 | 408 | 31 | 346 | IFN-α/β binding protein, SP | LSDV135 | 32 | 136R | 31 | M135R | 29 | B18R | 28 | ||
| SPV133 | 127790-128326 (179) | LSDV | AF325528 | 358 | 46 | 156 | A52R family protein | LSDV136 | 46 | 137R | 30 | M136R | 42 | K7R | 25 | ||
| SPV134 | 128372-129355 (328) | LSDV | AF325528 | 882 | 50 | 335 | E | LSDV137 | 50 | 138R | 39 | M137R | 39 | A51R | 33 | ||
| SPV135 | 129411-129974 (188) | MYX | AF170726 | 539 | 58 | 187 | A52R family protein | LSDV136 | 35 | 139R | 45 | M139R | 58 | A52R | 38 | ||
| SPV136 | 129994-131715 (574) | YLDV | AJ293568 | 1,317 | 44 | 564 | Kelch-like protein, TM | LSDV151 | 29 | 140R | 44 | M140R | 43 | A55R | 31 | ||
| SPV137 | 131727-132653 (309) | LSDV | AF325528 | 1,069 | 65 | 297 | Ser/Thr protein kinase, DNA replication | LSDV139 | 65 | 142R | 55 | M142R | 61 | B1R | 46 | ||
| SPV138 | 132666-133403 (246) | RFV | L26342 | 597 | 49 | 226 | N1R/p28-like host range RING finger protein | LSDV140 | 46 | 143R | 41 | M143R | 49 | ||||
| SPV139 | 133451-134146 (232) | MYX | AF170726 | 497 | 45 | 215 | EEV host range protein, TM | LSDV141 | 42 | 144R | 44 | M144R | 45 | C3L | 34 | ||
| SPV140 | 134184-135080 (299) | LSDV | AF325528 | 887 | 58 | 296 | Tyrosine protein kinase-like protein | LSDV143 | 58 | M147R | 48 | ||||||
| SPV141 | 135128-137032 (635) | LSDV | AF325528 | 1,515 | 46 | 627 | Ankyrin repeat protein | LSDV145 | 46 | 146R | 29 | M148R | 38 | B4R | 26 | ||
| SPV142 | 137100-138554 (485) | RFV | AF170722 | 1,183 | 46 | 484 | Ankyrin repeat protein, TM | LSDV147 | 43 | 146R | 38 | M149R | 44 | B4R | 24 | ||
| SPV143 | 138662-139951 (430) | LSDV | AF325528 | 778 | 39 | 426 | Ankyrin repeat protein | E | LSDV148 | 39 | 147R | 33 | M148R | 29 | B4R | 22 | |
| SPV144 | 140003-141481 (493) | LSDV | AF325528 | 776 | 34 | 492 | Ankyrin repeat protein | E | LSDV152 | 34 | 148R | 28 | M005 | 30 | B4R | 28 | |
| SPV145 | 141494-142453 (320) | L21931 | LSDV | AF325528 | 600 | 41 | 334 | Serpin | E | LSDV149 | 41 | 149R | 38 | M151R | 33 | C12L | 31 |
| SPV146 | 142522-143631 (370) | Q08520 | Human | AY016370 | 589 | 37 | 343 | GPCR, TM | LSDV011 | 37 | 145R/7L | 38 | M139R | 29 | |||
| SPV147 | 143683-143958 (92) | P32230 | LSDV | AF325528 | 217 | 46 | 91 | LSDV153 | 46 | 150R | 42 | M004.1 | 44 | ||||
| SPV148 | 144003-145022 (340) | P32231 | YLDV | AJ293568 | 539 | 38 | 335 | MHC class I α chain-like protein, SP, TM | E | 2L | 38 | ||||||
| SPV149 | 145132-145641 (170) | ShPV | P18388 | 195 | 39 | 113 | TM | LSDV155 | 39 | M003.2 | 29 | ||||||
| SPV150 | 145719-146168 (150) | LSDV | AF325528 | 498 | 62 | 145 | A52R family protein | LSDV156 | 65 | 6L | 41 | M003.1 | 45 | B15R | 41 | ||
Accession numbers are from the GenBank or SwissProtein database.
ShPV, sheeppox virus.
aa, amino acids.
Function was deduced either from the degree of similarity to known genes or from the presence of Prosite signatures. TM, a Z score of >1.96 was used for the prediction of transmembrane (TM) domains with the MEMSAT computer program (21). SP, N-terminal signal peptide (Z score of >3.5 within 40 amino acids of the N terminus determined using the SIGCLEAVE computer program [ftp://ftp.ebi.ac.uk/pub/software/unix/EMBOSS/] [43]).
Putative promoters (E, early; I, intermediate; L, late) were identified as previously described (2).
Best-matching ORF from the LSDV genome (accession no. AF325528).
Best-matching ORF from the YLDV genome (accession no. AJ293568).
Best-matching ORF from the MYX genome (accession no. AF170726).
Best-matching ORF from the VV Copenhagen genome (accession no. M35027), with the exception of the 8.9-kDa protein (*) (accession no. P07608), which corresponds to the VV Ankara strain.
Percentage of amino acid identity in the BLASTP2 analysis.
Gene families and duplicated genes with probable host range functions include those for extracellular enveloped virus proteins (EEV) (SPV119 and SPV120), ankyrins (SPV141, SPV142, SPV143, and SPV144), kelch-like proteins (SPV006, SPV015, and SPV136), A52-like proteins (SPV001, SPV007, SPV133, SPV135, and SPV150), G protein-coupled receptors (SPV005 and SPV146), major histocompatibility complex (MHC) class I α chain-like proteins (SPV003 and SPV148), and proteins of unknown function (SPV002, SPV004, SPV147, and SPV149).
Nucleic acid biogenesis, virion structure, and virion assembly.
SWPV contains most of the conserved poxviral genes involved in basic replicative functions, including 26 genes encoding RNA polymerase subunits; mRNA transcription initiation, elongation, and termination factors; and enzymes which direct posttranscriptional processing of viral mRNA (28) (Table 1). Also present in SWPV are seven ChPV homologues necessary for, or potentially involved in, DNA replication (SPV036, SPV074, SPV079, SPV080, SPV109, SPV130, and SPV137) (28).
SWPV nucleotide metabolism genes are similar to those found in the capripoxviruses and leporipoxviruses, except for the addition in SWPV of the large subunit of ribonucleotide reductase (6, 42, 44). These SWPV proteins potentially include homologues of thymidine kinase, dUTP pyrophosphatase, and a large and a small subunit of ribonucleotide reductase (Table 1). Interestingly, the large subunit of ribonucleotide reductase is absent from lumpy skin disease virus (LSDV), myxoma virus (MYX), and rabbit fibroma virus (RFV), all of which are closely related viruses (Table 1 and Fig. 2).
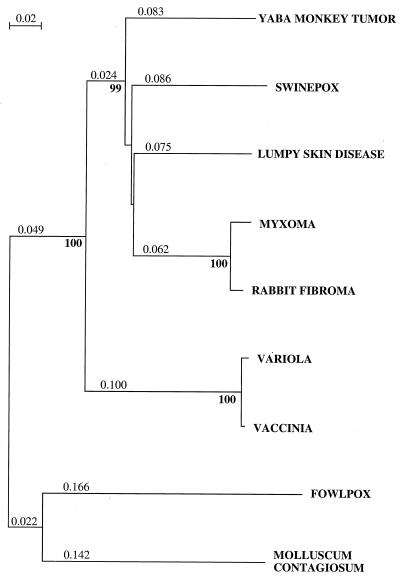
FIG. 2.
Comparison of SPV068 to ChPV RNA polymerase subunit RPO147. Proteins were aligned with ClustalW. Complete amino acid sequences were used to generate the unrooted tree with Melanoplus sanguinipes entomopoxvirus RPO147 as the outgroup. The neighbor-joining algorithm with Poisson correction for multiple substitution and 1,000 bootstraps was used as implemented by the Phylip package (15). The figure represents the ChPV subtree. Bootstrap values greater than 80% are in boldface. The bar indicates changes per 100 amino acids. Similar results were obtaining using maximum-likelihood and maximum-parsimony analysis (data not shown).
SWPV encodes 35 homologues of conserved poxviral structural proteins and those involved in virion morphogenesis and assembly (Table 1). These include proteins present in the virion core; proteins present in the intracellular mature virus (IMV) and associated membranes; potential enzymes involved in protein modification, DNA packaging, and redox activity; and at least four VV proteins found in or associated with the release of EEV (Table 1). SWPV, like LSDV, molluscum contagiosum virus, and fowlpox virus, lacks an obvious homologue of the VV IMV membrane protein D8L, a cell surface binding protein which is present in leporipoxviruses.
Host-related functions.
SWPV contains genes which likely function in modulation or evasion of host immune responses, modulation or inhibition of host cell apoptosis, or aspects of cell or tissue tropism. Many potential SWPV host range genes are homologues to genes present in other poxviruses. However, SWPV does contain a unique complement of these genes which likely dictate specific host range properties.
Some SWPV proteins are potentially secreted and are likely involved in the disruption or modulation of host immune responses as indicated by their similarity to other secreted immunomodulators and by the presence of potential signal peptide sequences. These include homologues of the gamma interferon (IFN-γ) receptor (SPV008), IFN-α/β binding protein (SPV132), and interleukin-18 (IL-18) binding protein (SPV011) (Table 1).
SWPV also contains predicted membrane-localized immunomodulatory proteins, including two homologues of G-protein coupled CC chemokine receptors (GPCR), and a CD47 homologue (6, 24, 25, 33) (Table 1). SPV005 is a truncated form of a GPCR lacking the first extracellular domain, while SPV146 resembles the complete receptor. SPV003 and SPV148 resemble cellular MHC class I α chain-like proteins, the molluscum contagiosum virus MC80R and yaba-like disease virus (YLDV) 2L gene products. These SWPV gene products have α1, -2, and -3 domains, one RGD motif, and three of the four cysteine residues required for formation of disulfide bonds. They differ from the MC80R gene product in that they lack the 50-amino-acid amino-terminal extension. SWPV MHC gene products also lack similarity to the transmembrane and cytoplasmic domains of cellular MHC homologues. In human and mouse cytomegaloviruses, another viral MHC homologue has been shown to interfere with NK cell-mediated clearance; however, the MC80R gene product appears to differ from herpesvirus homologues in the kinetics of complex formation and intracellular protein localization (36). The apparent lack of similarities in protein domains suggests that SWPV MHC-like genes may have other functions. SPV009 is similar to the leukemia-associated protein domain (LAP) and plant homeobox domain (PHD) finger protein found in LSDV, YLDV, and leporipoxviruses. Similar proteins in gammaherpesviruses are known to down-regulate expression of cellular MHC and NK cell activation ligands (20, 31, 40)
Several SWPV proteins may have intracellular immune modulation or immune evasion functions. These include homologues of VV double-stranded RNA-dependent protein kinase inhibitors (SPV010 and SPV032) which confer resistance to the antiviral effects of IFN (Table 1). Poxviral serine proteinase inhibitors (serpins) are known to perform anti-inflammatory roles, and the single serpin encoded in SWPV (SPV145) is similar to LSDV 149, YLDV 149R, and MYX M151R (25). Notably, SPV001, SPV007, SPV133, SPV135, and SPV150 are similar to members of the poxviral gene family which includes VV A52R (family 5 [38]) (data not shown). Although the functions of most of these genes are not known, VV A52R has been shown to function as an antagonist for host cell IL-1 receptor (IL-1R) and Toll-like receptor-mediated intracellular signaling and IL-18R-mediated induction of NF-κβ activation (5). The potential for IL-1 or Toll-like receptor inhibition by a family of SWPV proteins is significant considering the role of IL-1 or Toll-like receptor signaling in induction of innate immune and inflammatory responses (13).
SWPV encodes homologues of several other poxviral proteins known to affect virus virulence, virus growth in specific cell types, and/or cellular apoptotic responses (Table 1). These include homologues of VV C7L host range (SPV064) and A14.5L virulence (SPV103) proteins, the MYX M011L apoptosis regulator protein (SPV012), a serpin homologue (SPV145), and RFV N1R (ectromelia virus p28 host range factor) (SPV138). SWPV also encodes four proteins containing ankyrin motifs (SPV141 to SPV144) (Table 1). Poxviral ankyrin genes have been associated with host range functions in MYX, cowpox virus, and VV and may inhibit virally induced apoptosis (16, 29, 39). It has been suggested that specific complements of ankyrin genes dictate poxvirus host range, and the same is probably true for SWPV (3, 37).
SWPV has homologues of poxvirus genes resembling those for cellular enzymes (Table 1). SPV128, SPV129, and SPV140 resemble hydroxysteroid dehydrogenase, copper-zinc superoxide dismutase, and tyrosine protein kinase, respectively.
SWPV encodes several homologues of poxvirus proteins of unknown function, including the VV 8.9-kDa protein (SPV090), which interacts with VV morphogenesis proteins, and the variola virus (VAR) B22R putative membrane protein (SPV131) (Table 1) (27). SPV006, SPV015, and SPV136 are similar the Drosophila kelch protein and other poxvirus kelch-like proteins (Table 1). SPV018, SPV019, and SPV020 lack homology to other known genes.
Comparison of SWPV to other ChPVs.
SWPV is very similar to other ChPVs in overall genome structure and composition, as indicated by the presence of a central conserved core of 106 genes surrounded by regions containing many genes with apparent host range functions and the ITRs. The SWPV genome is highly colinear with the genomes of other ChPVs (Table 1) (2, 17, 35, 44). In comparison with VV, eight genes are absent in the SWPV central core. These include homologues of E11L (virion component), D8L (similar to carbonic anhydrase), A25L and A26L (A-type inclusion proteins), O2L (glutaredoxin), A31R, E5R, and E7R. A homologue of VV gene C7L (SPV064), which is believed to encode a host range factor, has been inserted between SPV063 and SPV065 (J2R and J3R homologues). Colinearity decreases toward the genome ends and disappears at the ITR. The SWPV left and right noncolinear regions contain only 44 genes and lack most VV gene homologues found in HindIII restriction fragments B, C, K, N, M, and F.
Gene colinearity is most conserved compared to LSDV, YLDV, and the leporipoxviruses (Table 1). SWPV overall amino acid identity is highest to proteins of LSDV (60% average), followed by those of YLDV (57%) and the leporipoxviruses (57%). Phylogenetic analysis of all of the SWPV genes located in the conserved central region indicates a close relationship among suipoxviruses (SWPV), capripoxviruses (LSDV), leporipoxviruses (MYX and RFV), and yatapoxviruses (Yaba monkey tumor virus [YMTV]) and a clear separation of these from other vertebrate poxvirus genera (orthopoxviruses, avipoxviruses, and molluscipoxviruses) (Fig. 2 and data not shown). These four genera also share genomic features which are different from those of orthopoxviruses, molluscipoxviruses, and avipoxviruses. Most notable is the absence of VV homologues A25L, A26L, and A31R and the insertion of one to three copies of the VV C7L homologue between J2R and J3R.
The terminal genomic regions of SWPV encode many proteins with probable functions involving host range, virulence, and immune modulation. At the amino acid level, many of these SWPV proteins are less similar to other poxvirus homologues than are viral proteins encoded in the conserved central core region. Two SWPV proteins located in terminal regions (SPV005 and SPV146) are most similar to cellular proteins (Table 1). Although closely related, SWPV contains eight genes that are absent in LSDV, including those for MHC class I proteins (two copies), ribonucleotide reductase (large subunit), hydroxysteroid dehydrogenase, and an M013L homologue and the unique SWPV genes SPV018, SPV019, and SPV020. In contrast, 13 genes present in LSDV are absent in SWPV, including those for epidermal-like growth factor, IL-10, IL-1R, VV C4/C10 like protein, endoplasmic reticulum (ER)-localized apoptosis regulator, immunoglobulin domain Ox-2 like homologue, VV E5R, E11L, K4L, F8L, and N1L and the novel LSDV genes LSDV026 and LSDV161. The absence of these genes from SWPV may explain the relatively reduced virulence and narrow host range of this virus.
Conclusions.
The genome sequence of SWPV has been determined. The high degree of similarity in genomic organization, gene content, and amino acid composition to viruses from the Suipoxvirus, Capripoxvirus, Yatapoxvirus, and Leporipoxvirus genera indicates a close structural and functional relationship among these genera. Although the highest conservation occurs in genes involved in basic replicative functions, including mRNA biogenesis, DNA replication, and virion structure and assembly, significant similarities exist among genes performing other functions. A unique complement of SWPV genes in the terminal genomic regions likely function in virulence, host range, and immune evasion. The relative paucity of SWPV genes with known host range function in other poxviruses may be responsible for the low pathogenicity of this virus in swine and for its narrow host range. An improved understanding of SWPV-host interactions will permit the engineering of novel vaccine viruses and expression vectors with enhanced efficacy and greater versatility.
Acknowledgments
We thank A. Zsak and A. Ciupryk for excellent technical assistance.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73:533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365–396. [DOI] [PubMed] [Google Scholar]
- 4.Barcena, J., M. M. Lorenzo, J. M. Sanchez-Puig, and R. Blasco. 2000. Sequence and analysis of a swinepox virus homologue of the vaccinia virus major envelope protein P37 (F13L). J. Gen. Virol. 81:1073–1085. [DOI] [PubMed] [Google Scholar]
- 5.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O’Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, C., S. Hota-Mitchell, L. Chen, J. Barrett, J. X. Cao, C. Macaulay, D. Willer, D. Evans, and G. McFadden. 1999. The complete DNA sequence of myxoma virus. Virology 264:298–318. [DOI] [PubMed] [Google Scholar]
- 7.Cheville, N. F. 1966. The cytopathology of swine pox in the skin of swine. Am. J. Pathol. 49:339–352. [PMC free article] [PubMed] [Google Scholar]
- 8.Datt, N. S. 1964. Comparative studies of pigpox and vaccinia viruses. I. Host range pathogenicity. J. Comp. Pathol. 74:62–69. [DOI] [PubMed] [Google Scholar]
- 9.De Boer, G. F. 1975. Swinepox, virus isolation, experimental infections and the differentiation from vaccinia virus infections. Arch. Virol. 49:141–150. [DOI] [PubMed] [Google Scholar]
- 10.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewing, B., L. Hillier, M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 8:175–185. [DOI] [PubMed] [Google Scholar]
- 12.Feller, J. A., R. F. Massung, P. C. Turner, E. P. Gibbs, E. O. Bockamp, A. Beloso, A. Talavera, E. Vinuela, and R. W. Moyer. 1991. Isolation and molecular characterization of the swinepox virus thymidine kinase gene. Virology 183:578–585. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald, K. A., and L. A. O’Neill. 2000. The role of the interleukin-1/Toll-like receptor superfamily in inflammation and host defence. Microbes Infect. 2:933–943. [DOI] [PubMed] [Google Scholar]
- 14.Foley, P. L., P. S. Paul, R. L. Levings, S. K. Hanson, and L. A. Middle. 1991. Swinepox virus as a vector for the delivery of immunogens. Ann. N.Y. Acad. Sci. 646:220–222. [DOI] [PubMed] [Google Scholar]
- 15.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biol. Sci. 12:543–554. [DOI] [PubMed] [Google Scholar]
- 16.Gillard, S., D. Spehner, R. Drillien, and A. Kirn. 1986. Localization and sequence of a vaccinia virus gene required for multiplication in human cells. Proc. Natl. Acad. Sci. USA 83:5573–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goebel, S. J., G. P. Johnson, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1990. The complete DNA sequence of vaccinia virus. Virology 179:247–266. [DOI] [PubMed] [Google Scholar]
- 18.House, J. A., and C. A. House. 1994. Swine pox, p.358–361. In A. D. Leman, B. E. Straw, W. L. Mengeling, S. D’Allaire, and D. J. Taylor (ed.), Diseases of swine, 7th ed. Iowa State University Press, Ames.
- 19.Huang, X., and A. Madan. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9:868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity 13:365–374. [DOI] [PubMed] [Google Scholar]
- 21.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1994. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 33:3038–3049. [DOI] [PubMed] [Google Scholar]
- 22.Kasza, L., and R. A. Griesemer. 1962. Experimental swine pox. Am. J. Vet. Res. 23:443–450. [PubMed] [Google Scholar]
- 23.Koonin, E. V., R. L. Tatusov, and K. E. Rudd. 1996. Protein sequence comparison at genome scale. Methods Enzymol. 266:295–322. [DOI] [PubMed] [Google Scholar]
- 24.Lalani, A. S., J. Masters, W. Zeng, J. Barrett, R. Pannu, H. Everett, C. W. Arendt, and G. McFadden. 1999. Use of chemokine receptors by poxviruses. Science 286:1968–1971. [DOI] [PubMed] [Google Scholar]
- 25.Massung, R. F., V. Jayarama, and R. W. Moyer. 1993. DNA sequence analysis of conserved and unique regions of swinepox virus: identification of genetic elements supporting phenotypic observations including a novel G protein-coupled receptor homologue. Virology 197:511–528. [DOI] [PubMed] [Google Scholar]
- 26.Massung, R. F., and R. W. Moyer. 1991. The molecular biology of swinepox virus. I. A characterization of the viral DNA. Virology 180:347–354. [DOI] [PubMed] [Google Scholar]
- 27.McCraith, S., T. Holtzman, B. Moss, and S. Fields. 2000. Genome-wide analysis of vaccinia virus protein-protein interactions. Proc. Natl. Acad. Sci. USA 97:4879–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss, B. 1996. Poxviridae: the viruses and their replication, p.2637–2671. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 29.Mossman, K., S. F. Lee, M. Barry, L. Boshkov, and G. McFadden. 1996. Disruption of M-T5, a novel myxoma virus gene member of poxvirus host range superfamily, results in dramatic attenuation of myxomatosis in infected European rabbits. J. Virol. 70:4394–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munz, E., and K. Dumbell. 1994. Swinepox, p.627–629. In J. A. W. Coetzer, G. R. Thomson, and R. C. Tustin (ed.), Infectious diseases of livestock, vol. 1. Oxford University Press, New York, N.Y.
- 31.Nicholas, J., V. Ruvolo, J. Zong, D. Ciufo, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1997. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J. Virol. 71:1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, J., and S. A. Teichmann. 1998. DIVCLUS: an automatic method in the GEANFAMMER package that finds homologous domains in single- and multi-domain proteins. Bioinformatics 14:144–150. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson, C. M., J. E. Parkinson, M. Hollinshead, and G. L. Smith. 1996. Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca2+ influx into infected cells. J. Virol. 70:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnitzlein, W. M., and D. N. Tripathy. 1991. Identification and nucleotide sequence of the thymidine kinase gene of swinepox virus. Virology 181:727–732. [DOI] [PubMed] [Google Scholar]
- 35.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of Molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19–42. [DOI] [PubMed] [Google Scholar]
- 36.Senkevich, T. G., and B. Moss. 1998. Domain structure, intracellular trafficking, and B2-microglobulin binding of a major histocompatibility complex class I homolog encoded by Molluscum Contagiosum virus. Virology 250:397–407. [DOI] [PubMed] [Google Scholar]
- 37.Shchelkunov, S. N., P. F. Safronov, A. V. Totmenin, N. A. Petrov, O. I. Ryazankina, V. V. Gutorov, and G. J. Kotwal. 1998. The genome sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology 243:432–460. [DOI] [PubMed] [Google Scholar]
- 38.Smith, G. L., Y. S. Chan, and S. T. Howard. 1991. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J. Gen. Virol. 72:1349–1376. [DOI] [PubMed] [Google Scholar]
- 39.Spehner, D., S. Gillard, R. Drillien, and A. Kirn. 1988. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J. Virol. 62:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. USA 97:8455–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripathy, D. N. 1999. Swinepox virus as a vaccine vector for swine pathogens. Adv. Vet. Med. 41:463–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2001. Genome of lumpy skin disease virus. J. Virol. 75:7122–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willer, D. O., G. McFadden, and D. H. Evans. 1999. The complete genome sequence of shope (rabbit) fibroma virus. Virology 264:319–343. [DOI] [PubMed] [Google Scholar]
- 45.Williams, P. P., M. R. Hall, and M. D. McFarland. 1989. Immunological responses of cross-bred and in-bred miniature pigs to swine poxvirus. Vet. Immunol. Immunopathol. 23:149–159. [DOI] [PubMed] [Google Scholar]