Abstract
1. The pharmacokinetics of cyclosporine were investigated before renal transplantation in 20 children aged 1.1 to 16.8 years. Cyclosporine was given as a single oral dose (10 mg kg-1) or as a 4 h i.v. infusion (3 mg kg-1). 2. The blood drug concentration was measured by both specific and nonspecific monoclonal radioimmunoassays. 3. The mean oral availability of cyclosporine was 20.6% (range 10.8-34.1%). 4. The mean ratio of AUCs measured by nonspecific and specific r.i.a. was 1.96 (range 1.4-2.7) after oral administration and 1.43 (range 1.1-2.0) after i.v. administration. The mean difference between the ratios was 38.5% (P = 0.0001). The ratio of AUCnonspecific to AUCspecific was inversely related to blood drug clearance (r = 0.57; P = 0.009). 5. The findings are suggestive of presystemic, pre-hepatic metabolism of cyclosporine which could contribute to the low, and highly variable bioavailability of this drug.
Full text
PDF
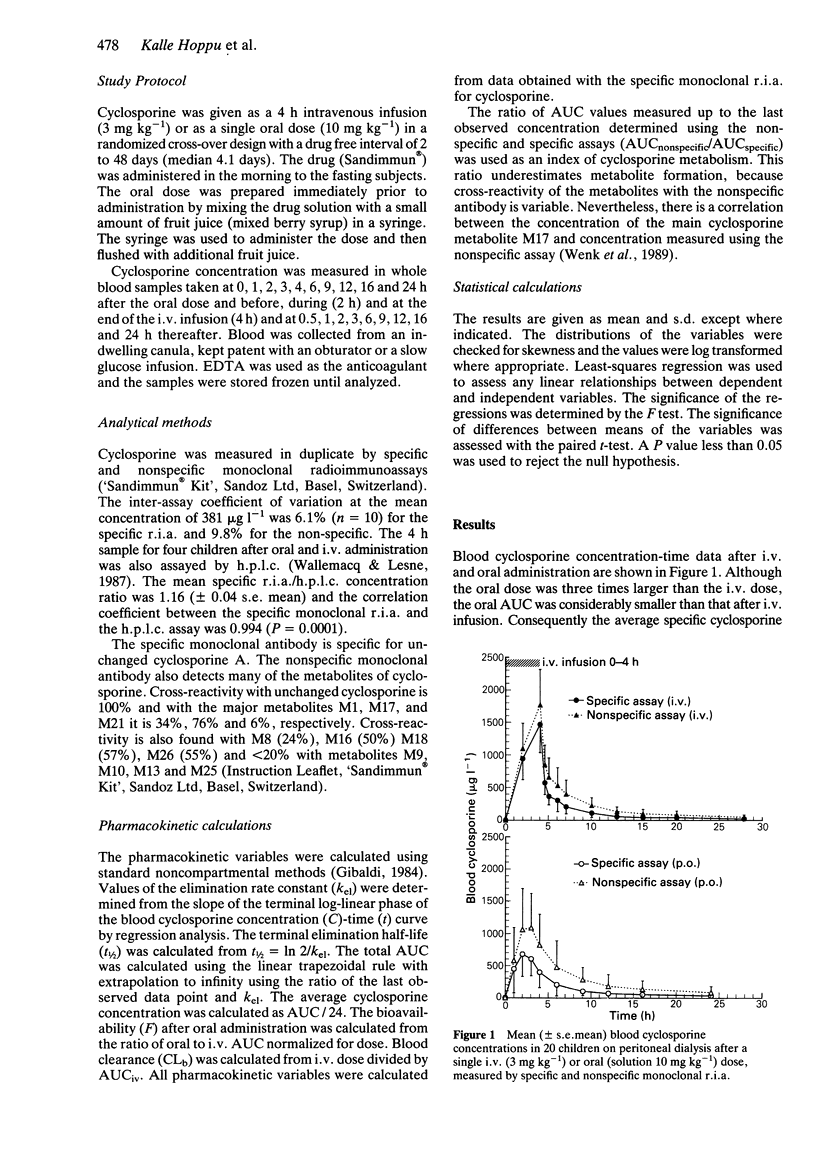
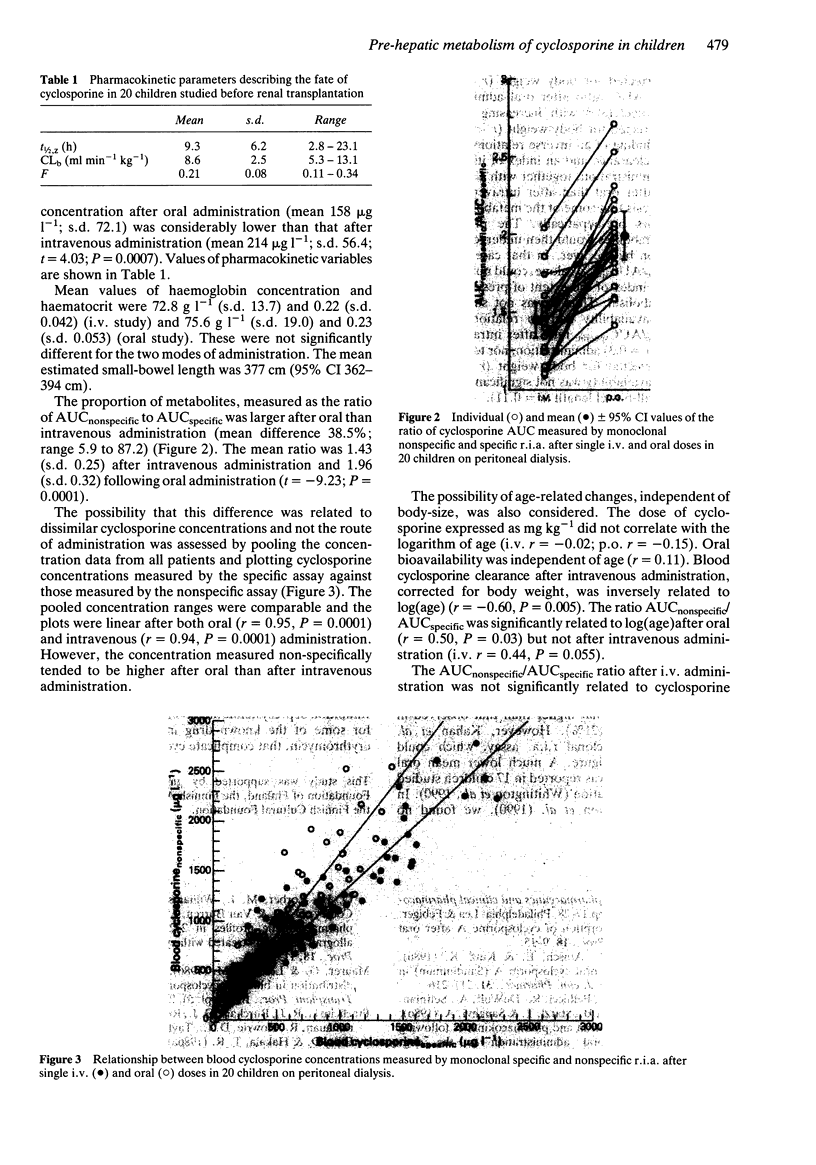


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grevel J. Absorption of cyclosporine A after oral dosing. Transplant Proc. 1986 Dec;18(6 Suppl 5):9–15. [PubMed] [Google Scholar]
- Grevel J., Nüesch E., Abisch E., Kutz K. Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects. Eur J Clin Pharmacol. 1986;31(2):211–216. doi: 10.1007/BF00606661. [DOI] [PubMed] [Google Scholar]
- Gridelli B., Scanlon L., Pellicci R., LaPointe R., DeWolf A., Seltman H., Diven W., Shaw B., Starzl T., Sanghvi A. Cyclosporine metabolism and pharmacokinetics following intravenous and oral administration in the dog. Transplantation. 1986 Mar;41(3):388–391. doi: 10.1097/00007890-198603000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B. D., Kramer W. G., Wideman C. A., Frazier O. H., Lorber M. I., Williams C., Flechner S. M., Cooley D. A., Van Buren C. T. Analysis of pharmacokinetic profiles in 232 renal and 87 cardiac allograft recipients treated with cyclosporine. Transplant Proc. 1986 Dec;18(6 Suppl 5):115–119. [PubMed] [Google Scholar]
- Maurer G., Lemaire M. Biotransformation and distribution in blood of cyclosporine and its metabolites. Transplant Proc. 1986 Dec;18(6 Suppl 5):25–34. [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Burckart G. J. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986 Mar-Apr;11(2):107–132. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- Siebert J. R. Small-intestine length in infants and children. Am J Dis Child. 1980 Jun;134(6):593–595. doi: 10.1001/archpedi.1980.02130180051015. [DOI] [PubMed] [Google Scholar]
- Wallemacq P. E., Lesne M. New automated high-performance liquid chromatographic analysis of cyclosporin A and G in human serum. J Chromatogr. 1987 Jan 23;413:131–140. doi: 10.1016/0378-4347(87)80220-7. [DOI] [PubMed] [Google Scholar]
- Watkins P. B., Wrighton S. A., Schuetz E. G., Molowa D. T., Guzelian P. S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987 Oct;80(4):1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitington P. F., Emond J. C., Whitington S. H., Broelsch C. E., Baker A. L. Small-bowel length and the dose of cyclosporine in children after liver transplantation. N Engl J Med. 1990 Mar 15;322(11):733–738. doi: 10.1056/NEJM199003153221105. [DOI] [PubMed] [Google Scholar]


