FIG. 2.
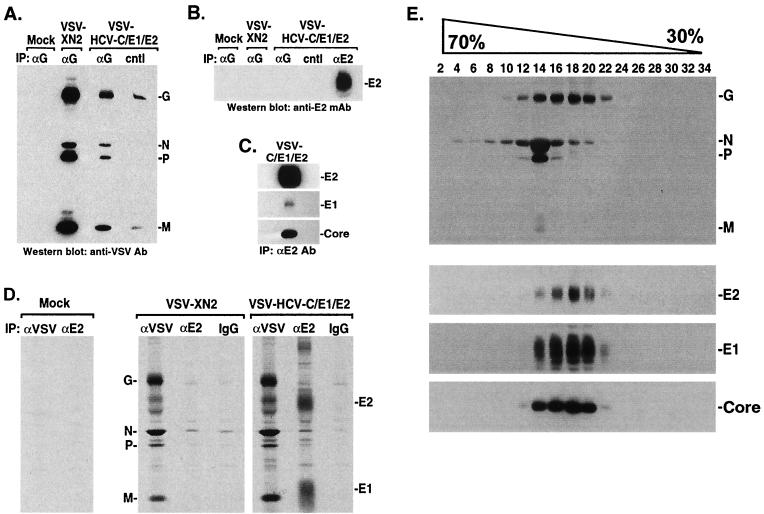
(A and B) HCV E2 is not associated with VSV-HCV-C/E1/E2. Cell medium from VSV-HCV-C/E1/E2- or control virus-infected cells (concentrated by ultracentrifugation) was immunoprecipitated (IP) with a sheep antibody (Ab) to VSV G (Biogenesis) or a goat antibody to HCV E2 (Immunodiagnostics Inc.). After SDS-PAGE, protein complexes were immunoblotted against mouse antiserum raised to VSV (A). Membranes were reprobed using mouse antiserum to HCV E2 (M. Kohara), which emphasized the absence of E2 in VSV complexes (B). cntl represents protein G agarose, which was used as a negative control. (C) Gradient-purified HCV Core, E1, and E2 form complexes. Sucrose gradient fractions containing HCV-LPs were identified by immunoblot and were immunoprecipitated using E2-specific antibody (Immunodiagnostics Inc.). Complexes were washed, analyzed by SDS-PAGE, and immunoblotted using antibody to HCV Core (Biogenesis), E1 (H. Greenberg), and E2 (M. Kohara). (D) Coimmunoprecipitation analysis of VSV-HCV-C/E1/E2-infected cells. [35S]methionine/cysteine-labeled lysates (600 μCi/12 h) from mock, VSV-XN2, or VSV-HCV-C/E1/E2 were immunoprecipitated with mouse antiserum raised to VSV or anti-E2 MAb (544; M. Kohara) or normal mouse immunoglobulin G. Complexes were washed, analyzed by SDS-PAGE, and visualized by autoradiography. (E) Analysis of HCV-LPs by sucrose gradients. BHK cells were infected at an MOI of 0.1 for 18 h and were then lysed in 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 0.5 mM EDTA. The lysates were clarified by centrifugation through 30% sucrose for 6 h at 150,000 × g. The resulting pellets were layered onto a continuous 30 to 70% sucrose gradient. One-milliliter fractions were collected, centrifuged, and analyzed by SDS-PAGE and immunoblotting using antibody to VSV (top gel) or HCV Core (Biogenesis), E1 (S. Polyak), or E2 (C. Rice) (bottom three gels). HCV structural proteins colocalize in fractions 14 to 20 and are separable from VSV proteins.