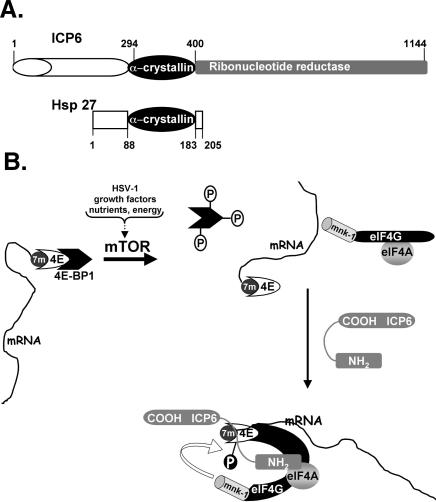
Figure 9.
Model illustrating how ICP6 promotes eIF4F complex assembly and eIF4E phosphorylation. (A) ICP6 has an α-crystallin domain with homology to the cellular chaperone, hsp27. ICP6 is a multifunctional polypeptide with a C-terminal domain (residues 400–1144, shaded in gray) that specifies one subunit of the virus-encoded ribonucleotide reductase holoenzyme (Goldstein and Weller 1988a,b). The α-crystallin homology domain (residues 294–400) present in ICP6 (Chabaud et al. 2003) and hsp27 (residues 88–183) is shown as a black oval. (B) The cellular cap-binding protein is depicted bound to the 7-methyl GTP cap (7m) at the mRNA 5′ end. eIF4E, in turn, is bound to the translational repressor 4E-BP1 and is unable to assemble into an eIF4F complex with the other translation initiation factors, eIF4G and eIF4A. Activation of the kinase mTOR in response to a variety of cues such as HSV-1 infection, growth factor signaling, alterations to the nutrient pool, or changes in cellular energy reserves results in phosphorylation of the translational repressor protein 4E-BP1 and the release of eIF4E from 4E-BP1. However, the release of eIF4E from the repressor 4E-BP1 is not sufficient for eIF4F assembly. In growth-arrested cells infected with HSV-1, the assembly of eIF4E together with eIF4G and eIF4A into an eIF4F complex requires the assistance of ICP6. Through its N-terminal domain, we propose that ICP6 recognizes eIF4F components and potentially acts as a chaperone to foster the assembly of an eIF4F complex in HSV-1-infected cells. The molecular architecture of this complex allows for the correct apposition of the cellular kinase mnk with its substrate, eIF4E, and its assembly facilitates eIF4E phosphorylation.