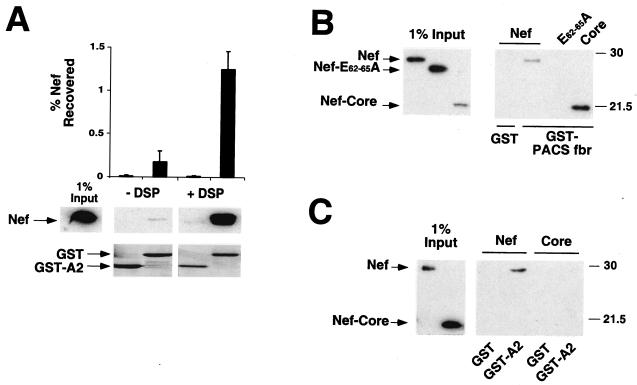
FIG. 5.
HIV-1 Nef directly binds to the cytoplasmic domain of HLA-A2 in vitro. (A) Nef association with the HLA-A2 cytoplasmic tail is specifically stabilized by cross-linking. The cytoplasmic tail of HLA-A2 (A2) and HIV-1 Nef were overexpressed and purified from Escherichia coli as GST fusion proteins. GST was cleaved from Nef by using thrombin, as described in Materials and Methods. Purified GST and GST-A2 were then incubated with recombinant Nef, with or without treatment with a cross-linking reagent (DSP), and proteins were then precipitated with glutathione beads. The mean percentage of Nef recovered in the pulldown assay was determined by densitometry of immunoblots from three independent experiments (graph). A representative anti-Nef immunoblot (middle panel) and a Coomassie stain of the membrane (bottom panel) are shown. All images in the middle panel were taken from the same exposure of an anti-Nef immunoblot. (B) Nef core domain is functional for binding to GST-PACSfbr. The furin binding region (fbr) of human PACS-1a (amino acids 117 to 266) was fused to GST, purified, and tested for in vitro binding to either NL4-3 Nef, a Nef acidic domain mutant (E62-65A), or the Nef core domain. Results shown are representative of two independent experiments, each performed in triplicate. (C) The Nef core domain (amino acids 58 to 206) is not sufficient to bind GST-A2. GST pulldown assays were performed as described above. A fraction of Nef protein input (1%) was immunoblotted (left panel) to control for equal protein concentration and antibody recognition. Results shown are representative of three independent experiments, each performed in duplicate.