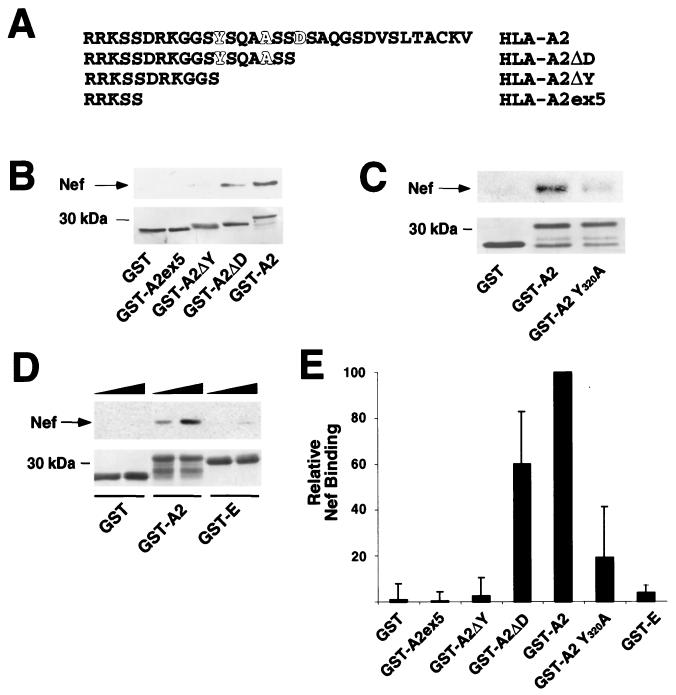
FIG. 6.
HIV-1 Nef directly binds to a sequence in the cytoplasmic domain of MHC-I that is necessary for downmodulation. (A) Cytoplasmic tail sequences of deletion mutants used in Nef binding assays. Amino acids that are important for downmodulation of MHC-I by Nef are highlighted (4, 22). (B) Nef binds to a region of the cytoplasmic tail of HLA-A2 that contains amino acid residues required for downmodulation. C-terminal truncations of the HLA-A2 cytoplasmic domain were fused to GST and subjected to the in vitro binding assay described in the legend to Fig. 5. A representative immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. (C) Tyrosine 320 is required for efficient Nef binding. A point mutation in the cytoplasmic tail of HLA-A2 (GST-A2 Y320A) was tested in the in vitro binding assay. A representative immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. (D) The cytoplasmic domain of HLA-E does not efficiently bind Nef. The cytoplasmic tail of HLA-E was fused to GST (GST-E) and was tested in the in vitro binding assay. An anti-Nef immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. In this experiment, Nef protein was added at increasing amounts (1 to 2 μg), indicated by the solid triangles. (E) Quantitation of the ability of GST proteins to bind Nef in vitro. To assess the relative binding of the cytoplasmic tail constructs, the amount of Nef coprecipitating was determined by densitometry using immunoblots from a minimum of three independent experiments. Data presented are the mean percentages of wild-type binding ± standard deviations for each tail construct.