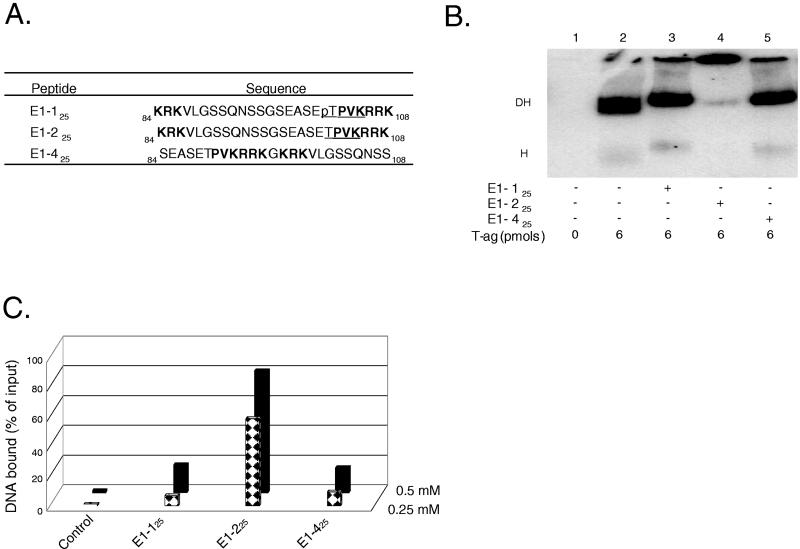
FIG. 2.
Studies conducted with CDK/NLS peptides derived from BPV E1. (A) Peptides derived from BPV E1 in the vicinity of the putative CDK/NLS element. Peptide numbering is based on the system used to designate residues in BPV E1. Peptides E1-125 and E1-225 both contain the bipartite NLS found in BPV E1; they differ in that peptide E1-125 contains a phosphate (“p”) on Thr102. The control peptide E1-425 was formed by swapping the residues normally found on either side of Gly96. Residues in boldface define the bipartite BPV E1 NLS, and underlined residues represent the putative recognition motif for the cyclin/Cdk kinase (38). (B) T-ag band shift reactions conducted in the presence of the BPV E1 CDK/NLS set of peptides. The reactions in lane 1 were conducted in the absence of either T-ag or peptide (input DNA is not shown). The reaction in lane 2 was conducted in the presence of T-ag (6 pmol). The reactions in lanes 3 to 5 were conducted in the presence of T-ag and 5 nmol of the indicated peptides. (C) Determination of whether peptides derived from the CDK/NLS region of BPV E1 bind to DNA. Nitrocellulose filter-binding assays, conducted under replication conditions, were performed with the indicated peptides and the 64-bp SV40 core origin-containing oligonucleotide. In this series of experiments, two different concentrations of peptide (0.25 and 0.5 mM) were used. The percentage of input DNA bound to a given filter was determined by scintillation counting. As a control, the percentage of input DNA bound to a nitrocellulose filter in the absence of peptide was also determined.