Figure 2.
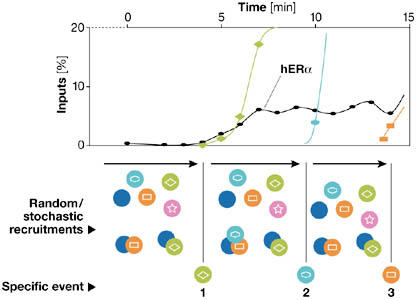
Proposed allosteric, stochastic and dynamic model. This model integrates the general concepts that have emerged from detecting oestrogen receptor-α (ER-α) in live-cell imaging experiments such as fluorescence recovery after photobleaching (FRAP) and those from chromatin immunoprecipitation (ChIP) assays. This model incorporates both stochastic and deterministic concepts into transcriptional attainment. We postulate that transcriptionally productive complexes, which have slower mobility, are rarely formed on promoters. Transcription initiation requires a specific sequence of events to occur, defining a transcriptional ratchet that orientates progression through the cycles. Before that one deterministic event takes place, many rapid stochastic and transient associations of factors occur that are unproductive. It is only when a specific required factor is recruited at the appropriate time that progress is made. Allostery is instrumental in these transitions, as functional, three-dimensional changes are anticipated to occur on all participating partners (namely, proteins, DNA and RNA). Whereas FRAP experiments mainly detect the rapid, unproductive binding of factors, ChIP assays allow the determination of the precise kinetics of the productive associations and of the time required for the transition from one step to another.
