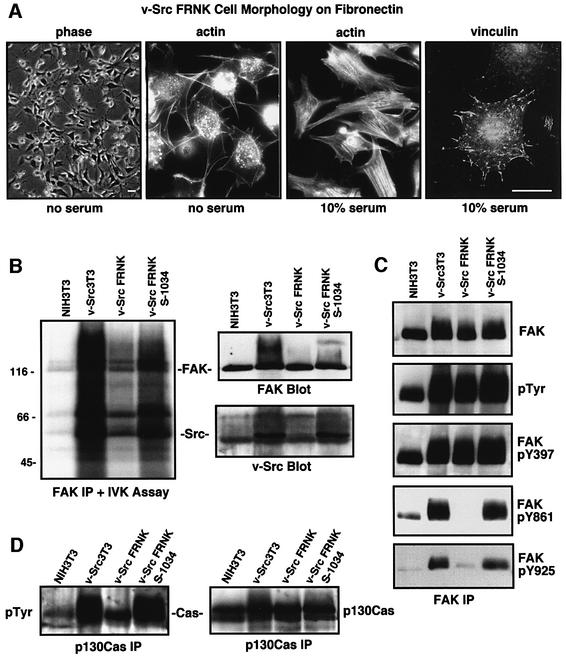
Fig. 3. FRNK inhibits the formation of a v-Src–FAK signaling complex, promotes FAK and p130Cas dephosphorylation, but does not affect cell morphology. (A) v-Src3T3 FRNK cells plated onto FN in the presence or absence of serum visualized by phase, actin staining with FITC–phalloidin or indirect staining for focal contact-associated vinculin. The scale bar is 30 µm. (B) FAK was immunoprecipitated (IP) from the indicated serum-starved cells and analyzed for 32P-associated in vitro kinase (IVK) activity. The FAK IPs were resolved by SDS–PAGE, transferred to a PVDF membrane and exposed to film. Equal levels of FAK and the co-immunoprecipitation of v-Src were visualized by blotting. (C) FAK IPs from the indicated serum-starved cells were analyzed sequentially with anti-phosphotyrosine (pTyr) or with phospho-specific antibodies to FAK Tyr397 (pY397), Tyr861 (pY861) or Tyr925 (pY925). FAK levels were determined by FAK blotting, and these analyses were performed on two sets of IPs. (D) p130Cas IPs from the indicated cells were analyzed sequentially by anti-P.Tyr and p130Cas blotting.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.