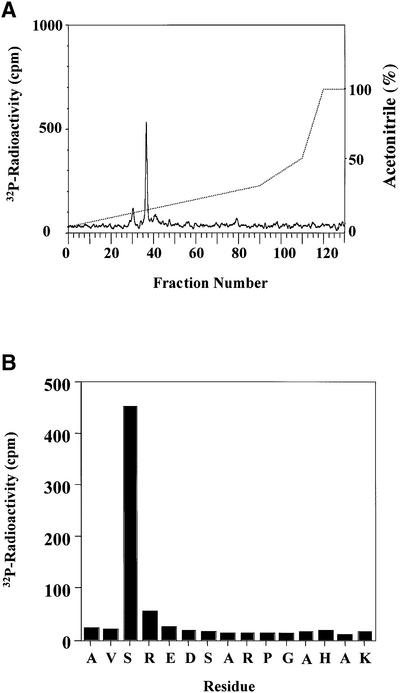
Fig. 3. Mapping of the site in hnRNP A0 phosphorylated by MAPKAP-K2. (A) Purified GST–hnRNP A0 was phosphorylated for 60 min in vitro with 150 U/ml of MAPKAP-K2 and 0.1 mM [γ-32P]ATP, and subjected to SDS–PAGE. The band corresponding to GST–hnRNP A0 was excised, digested with trypsin and the resulting peptides separated by reverse phase hydrophobic interaction chromatography on a C18 column equilibrated in 0.1% trifluoroacetic acid. 32P-radioactivity is shown by the full line, and the acetonitrile gradient by the broken line. (B) The mass of the major tryptic phosphopeptide from A (m/z = 1631.8) corresponds to that of a mono-phosphorylated form of a tryptic peptide comprising residues 82–96. The sequence of the peptide was confirmed by Edman sequencing and the site of phosphorylation was identified as the first serine in the peptide in a solid phase sequencing experiment. The methodology is detailed elsewhere (Stokoe et al., 1992).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.