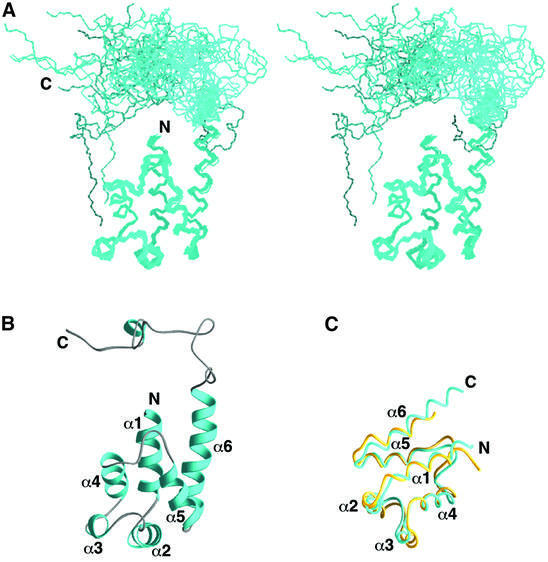
Fig. 1. Three-dimensional structure of PEA-15. (A) Stereoview of the backbone atoms (N, Cα and C′) of 20 NMR conformers of PEA-15 superimposed over residues 1–97. (B) Ribbon representation of the averaged minimized structure of PEA-15. The orientation of the structure is as shown in (A). Residues 2–14, 17–27, 33–37, 42–51, 61–69 and 73–89 comprise helices α1, α2, α3, α4, α5 and α6, respectively. The helices are colored in cyan, and the loops and C-terminal tail in gray. (C) Backbone representation of the death effector domains of PEA-15 (cyan) and FADD (yellow) (Eberstadt et al., 1998; PDB accession code 1A1Z), superimposed over the Cα atoms of residues 1–83.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.