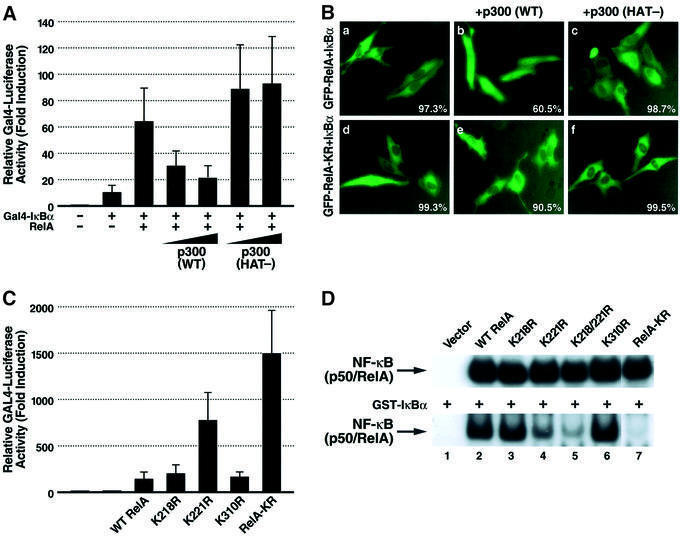
Fig. 5. Acetylation of lysine 221 in RelA plays a key role in regulating assembly with IκBα. (A) p300 inhibits the interaction of RelA with IκBα in a mammalian one-hybrid assay. Expression plasmids encoding wild-type RelA (0.1 µg) were co-transfected into 293T cells with plasmids encoding the GAL4 DNA binding domain fused to IκBα (pFA-IκBα) (1 ng) together with a GAL4 enhancer–luciferase reporter (0.1 µg) (pFR-luc) (Stratagene) in the presence of increasing amounts of either p300 or p300 (HAT-) mutant expression plasmids (100 ng and 200 ng, respectively). Luciferase activity was measured as in Figure 3A. (B) p300 prevents the cytoplasmic sequestration of wild-type RelA but not RelA-KR induced by co-expression of IκBα. HeLa cells were co-transfected with expression vectors encoding GFP–RelA (0.2 µg) or GFP–RelA-KR and IκBα (50 ng) together with wild-type p300 expression vector (1 µg). Note that in the presence of p300, the majority of GFP–RelA localizes in the nucleus while GFP–RelA-KR localizes predominantly in the cytoplasm. The percentage of cells displaying the depicted phenotype derived from inspection of at least 500 cells present in multiple fields in two independent experiments is presented in the bottom right corner of each panel. (C) The RelA-KR mutant displays a stronger interaction with IκBα in vivo. Interaction of IκBα with wild-type RelA or the lysine-to-arginine substitution mutants was tested in the mammalian one-hybrid assay system as in (A). (D) Whole-cell extracts from 293T cells co-transfected with expression vectors encoding wild-type RelA or the various RelA substitution mutants as indicated together with expression vectors encoding p50 and p300 were prepared and tested in EMSA. Binding of the nuclear complexes to DNA in the presence (lower panel) or absence (upper panel) of added recombinant of GST–IκBα (50 ng) is shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.