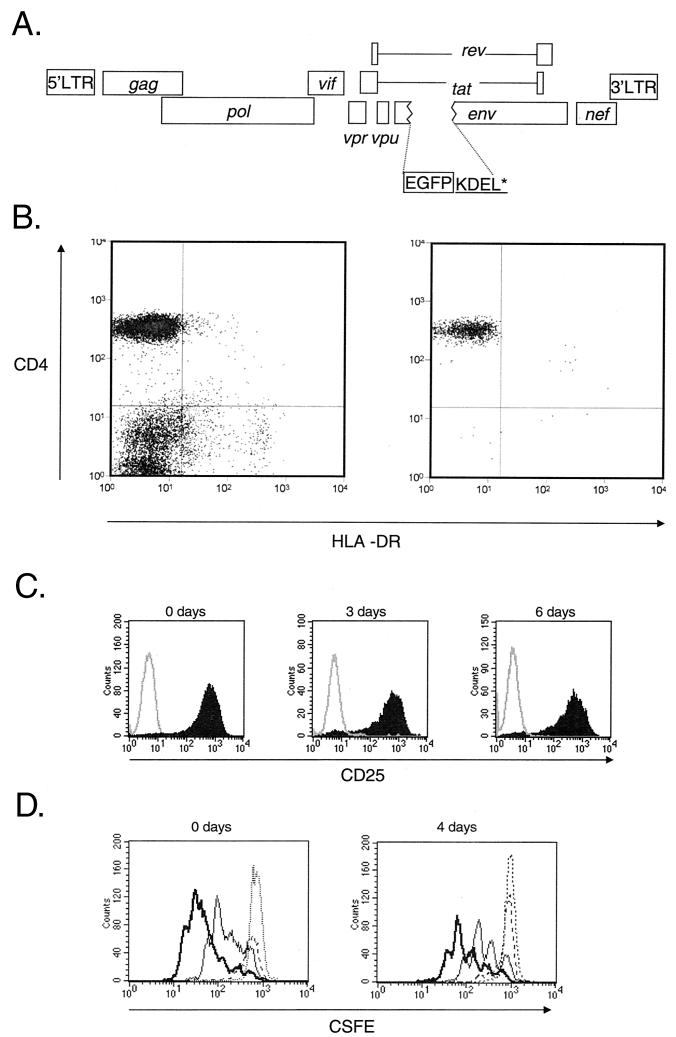
FIG. 6.
Analysis of the decay of the preintegration state of HIV-1 latency. (A) Map of reporter virus NL4-3-GFP used to monitor the fate of HIV-1 in infected resting CD4+ T cells. NL4-3-GFP contains the EGFP coding sequence inserted in frame in the env gene, followed by codons for the KDEL sequence to retain GFP in the ER and a stop codon. Highly purified resting CD4+ T cells were infected with VSV-G-pseudotyped NL4-3-GFP. Following infection, the cells were extensively washed and cultured for various times before being subjected to activation with mitogen PHA and irradiated allogeneic PBMC. Expression of GFP by the infected cells was monitored by flow cytometry. (B) Purity of resting CD4+ T-cell preparations. Resting CD4+ T cells were isolated from the peripheral blood of HIV-negative donors by magnetic bead depletion and cell sorting. Cells stained with antibodies to CD4 and activation marker HLA-DR before (left) and after (right) magnetic bead depletion and cell sorting were analyzed by flow cytometry. (C) Efficiency of in vitro activation of CD4+ T cells. Uninfected resting CD4+ T cells were activated with PHA and irradiated feeders immediately after isolation (0 days) or after 3 or 6 days of culture in MM. Cells were stained with an anti-CD25 antibody 72 h after activation. Solid histogram, CD25 staining; open histogram, isotype control. (D) Proliferative responses of resting CD4+ T cells following activation with PHA and irradiated allogeneic PBMC. Resting CD4+ T cells were stained by CFSE and activated immediately after isolation (left) and after 4 days of culture (right). CFSE intensity was measured at 24 (dotted line), 48 (broken line), 72 (thin line), and 96 h (thick line) after activation.