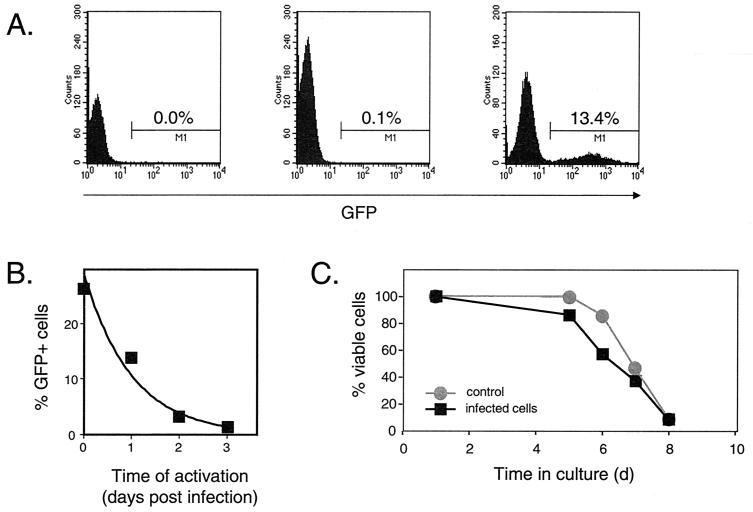
FIG. 7.
Analysis of preintegration latency. (A) Dependence of viral gene expression on cellular activation. Resting CD4+ T cells were left uninfected (left) or were infected with pseudotyped NL4-3-GFP virus at a multiplicity of infection (MOI) of 1 (center and right). Four days after infection, GFP expression was analyzed by flow cytometry. In the absence of an activating stimulus (center), the percentage of cells with green fluorescence 4 days after infection was very low (0.1%), only slightly higher than the background observed in uninfected cells (left). However, if the resting cells were activated with PHA and irradiated allogeneic PBMC on day 1 postinfection, a significant portion of infected CD4+ T cells expressed GFP on day 4 postinfection (right). (B) Decay of HIV-1 in the preintegration state. Resting CD4+ T cells were infected with pseudotyped NL4-3-GFP at a MOI of 7, washed, and maintained in RPMI 1640 supplied with 10% fetal calf serum (FCS) for the indicated times before activation with PHA and allogeneic PBMC. The decay of virus in the preintegration state was measured as a decrease in the percentage of GFP-positive cells determined by FACS analysis 48 to 72 h after activation. The experiment shown is representative of six independent experiments, which are summarized in Table 1. (C) Viability of resting CD4+ T cells infected with pseudotyped NL4-3-GFP. Highly purified resting CD4+ T lymphocytes were isolated and infected with pseudotyped NL4-3-GFP at a MOI of 2. Both HIV-1-infected cells and noninfected resting T cells were maintained in RPMI 1640 supplemented in 10% FCS. Cell viability was measured over time by exclusion of vital dye trypan blue.