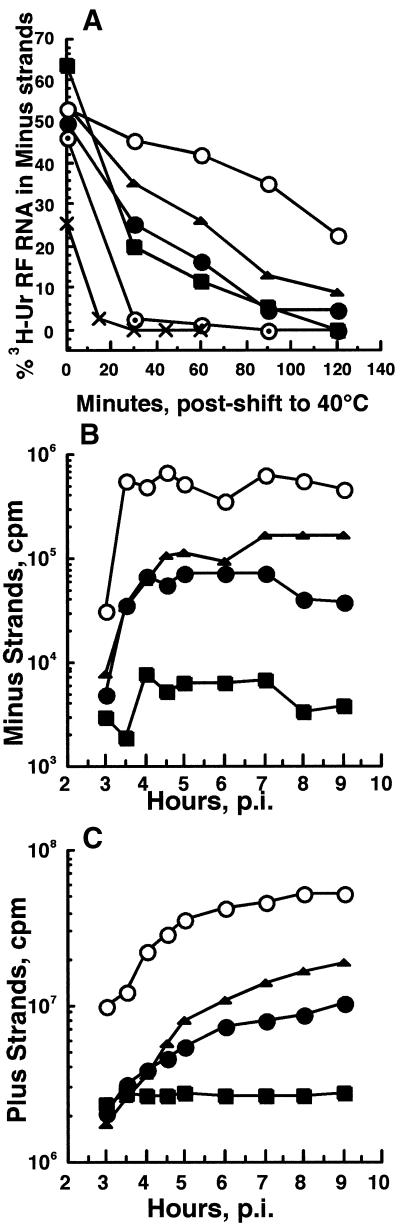
FIG. 4.
Effect of shift from 30 to 40°C on minus-strand and plus-strand synthesis. (A) Ability to continue minus-strand synthesis. CEF cultures were infected with Ser183 (▪), Ala183 (•), Lys183 (▴), ts4 (×), or Toto1101 (○) at an MOI of 100 at 30°C and were shifted to 40°C at 2 h p.i. (Toto1101 and ts4) or 3 h p.i. (Arg183 mutants), when ∼10% of each virus's 30°C maximal transcription rate was observed. Cultures maintained at 30°C were labeled at 30°C for 1 h beginning the hour before the time of shift-up (zero time point); cultures shifted to 40°C were labeled for 30-min periods beginning at the time of shift. Labeling was performed with 250 μCi of [3H]uridine/ml in the presence of 20 μg of AMD/ml. A duplicate set of Ala183-infected cultures was treated with 100 mg of cycloheximide/ml beginning at the time of shift to 40°C and labeled in the presence of the drug (⊙). Cells were harvested at the end of the pulse period, and the viral RF RNA was isolated and analyzed for its content of radiolabeled minus strands. These results are expressed as percentages of the total incorporation in the cores of RI-native RFs (active also in plus-strand synthesis) that was in minus-strand RNA (see Materials and Methods). (B) Accumulation of minus-strand RNA. The viral RF RNA was purified from the lysates analyzed in panel C, and their total amount of radiolabeled minus-strand RNA was determined in RNase protection assays, as described in Materials and Methods. (C) Accumulation of plus-strand RNA. CEF cells, infected with each virus at an MOI of 100 at 30°C, were shifted to 40°C at 1 h p.i. and labeled continuously with 250 μCi of [3H]uridine/ml in the presence of 20 μg of AMD/ml. Cells were harvested at the times indicated, and acid-precipitable incorporation into viral RNA per 3.7 × 106 cells was determined.