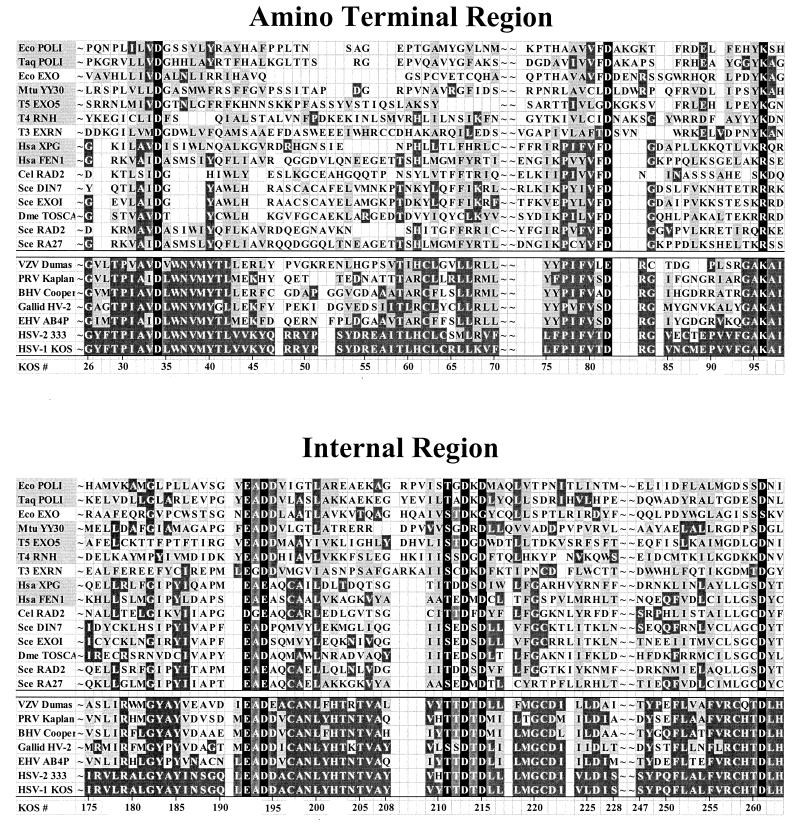
FIG. 2.
Sequences of the amino-terminal and internal domains of Vhs and selected cellular nucleases. The amino acid sequences of the amino-terminal and internal domains of the Vhs polypeptides from seven alphaherpesviruses are shown below the double line; the sequences of the corresponding domains of selected cellular and phage nucleases (listed in Table 1) are shown above the double line. The amino acid coordinates of the Vhs polypeptide from HSV-1 strain KOS are shown at the bottom of each panel. Amino acids that are identical to the residues in HSV-1 strain KOS are shown in white lettering on a dark grey background. Amino acids that represent a conservative change from the residues in HSV-1 strain KOS are shown in black lettering on a light grey background. The names of cellular and phage nucleases for which structural and/or genetic data have identified key residues that are in the active site are highlighted by a light grey background in the leftmost column. The active-site residues from these nucleases that are conserved in other cellular and phage nucleases and in the Vhs polypeptides are shown in white lettering on a black background. These residues were the focus of site-directed mutagenesis of the Vhs polypeptide (see Fig. 3 and 4). VZV, varicella-zoster virus; PRV, pseudorabies virus; BHV, bovine herpesvirus; Galid HV-2, Gallid herpesvirus 2; EHV, equine herpesvirus.