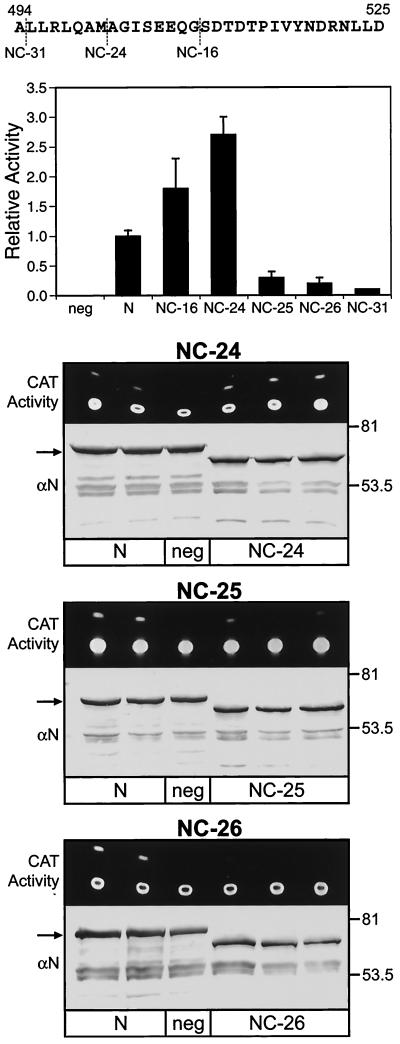
FIG. 1.
Effect of N protein C-terminal truncations on MV minireplicon CAT reporter gene expression. Duplicate or triplicate cell monolayers were transfected with plasmids directing the expression of minigenomic RNA and transcripts for P, L, and either parental N or N proteins lacking the C-terminal 16 (NC-16), 24 (NC-24), 25 (NC-25), 26 (NC-26), or 31 (NC-31) amino acids. The sequence of the C-terminal 32 amino acids of the 525-amino-acid Edmonston MV N protein is illustrated at the top. Cell extracts were processed separatelyfor measurement of CAT activity; results are expressed as an experimental average with correction for transfection efficiency, and that value was averaged for three trials. The latter average and standard error of the mean are presented in the histogram. Results were normalized to CAT activity supported by parental N protein, where that value equals 1.0; transfections with constructs expressing parental N protein were run in parallel with transfections with N protein truncation constructs. Raw data for one trial are presented for NC-24, NC-25, and NC-26. These data include the chromatogram for fluorescent CAT reaction products (i.e., substrate and monoacetylated product, UV illumination) and Western blot analysis of cell extracts with an anti-N protein monoclonal antibody (αN) as a probe. The positions of molecular size markers (kilodaltons) for the latter are indicated to the right. An arrow indicates the position of the 60-kDa N protein. The N protein truncation mutants exhibited greater electrophoretic mobility, corresponding to an apparent mass of 57 kDa, with degradation products migrating at between 45 and 55 kDa. Transfections lacking L constructs but expressing the template were used as negative controls (neg) for CAT activity.