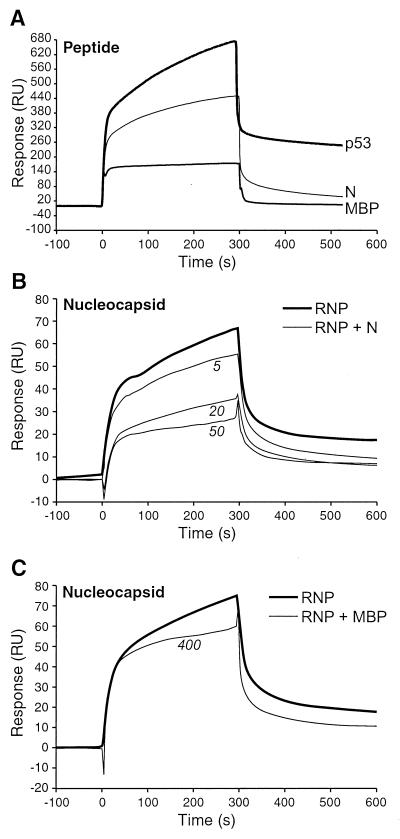
FIG. 4.
BIAcore3000 sensorgrams illustrating the binding of synthetic peptides to immobilized Hsp72 or peptide competitive inhibition of nucleocapsid-Hsp72 binding. (A) Binding assays. Solutions of peptides (1 mM) representing the Hsp72-interactive domain of p53 (p53), the C terminus of the MV N protein (N), or an immunogenic domain of myelin basic protein (MBP) were passed over sensor chips to which Hsp72 was conjugated. All peptides were 15 amino acids long. Retention of a peptide on the sensor chip was indicated by a change in RU over the course of the 300-s injection interval. Background peptide-sensor chip interactions were defined by the negative-control MBP peptide; these sensorgrams were identical to those of N peptide passed over negative-control sensor flow channels (data not shown). (B and C) Competitive inhibition assays. (B) MV ribonucleoprotein core particles (RNP or nucleocapsid) representing 1 nM N protein were passed over sensor chips in the presence or absence of N peptide. N peptide concentrations of 5, 20, and 50 μM decreased the magnitude of RNP binding to immobilized Hsp72 in a peptide dosage-dependent manner. (C) The same competitive inhibition experiment was performed to compare RNP binding to Hsp72 with or without the addition of the negative-control MBP peptide. Minimal inhibition was observed at peptide concentrations of as high as 400 μM.